
Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
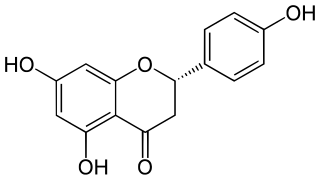
Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs.

Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone is hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.
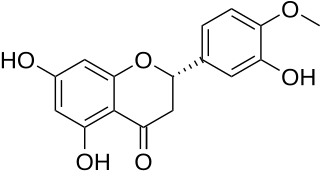
Hesperetin is the 4'-methoxy derivative of eriodictyol, a flavanone. Hesperetin's 7-O-glycoside, hesperidin, is a naturally occurring flavanon-glycoside, the main flavonoid in lemons and sweet oranges. Hesperetin are not found to a significant extent in Citrus spp.

Flavones are a class of flavonoids based on the backbone of 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one).
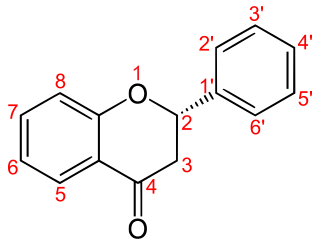
The flavanones, a type of flavonoids, are various aromatic, colorless ketones derived from flavone that often occur in plants as glycosides.

Chalcone synthase or naringenin-chalcone synthase (CHS) is an enzyme ubiquitous to higher plants and belongs to a family of polyketide synthase enzymes (PKS) known as type III PKS. Type III PKSs are associated with the production of chalcones, a class of organic compounds found mainly in plants as natural defense mechanisms and as synthetic intermediates. CHS was the first type III PKS to be discovered. It is the first committed enzyme in flavonoid biosynthesis. The enzyme catalyzes the conversion of 4-coumaroyl-CoA and malonyl-CoA to naringenin chalcone.
In enzymology, a flavanone 4-reductase (EC 1.1.1.234) is an enzyme that catalyzes the chemical reaction
In enzymology, a flavanone 3-dioxygenase (EC 1.14.11.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a flavone synthase (EC 1.14.11.22) is an enzyme that catalyzes the chemical reaction
In enzymology, a flavonoid 3'-monooxygenase (EC 1.14.14.82, was wrongly classified as EC 1.14.13.21 in the past) is an enzyme that catalyzes the chemical reaction

In enzymology, a chalcone isomerase is an enzyme that catalyzes the chemical reaction
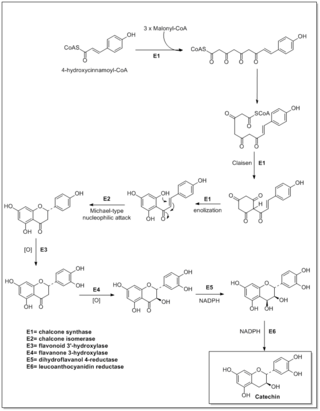
Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.

The flavan-4-ols (3-deoxyflavonoids) are flavone-derived alcohols and a family of flavonoids. Flavan-4-ols are colorless precursor compounds that polymerize to form red phlobaphene pigments. They can be found in the sorghum. Glycosides can be isolated from a methanol extract of the rhizomes of Abacopteris penangiana.

Xanthohumol is a natural product found in the female inflorescences of Humulus lupulus, also known as hops. This compound is also found in beer and belongs to a class of compounds that contribute to the bitterness and flavor of hops. Xanthohumol is a prenylated chalconoid, biosynthesized by a type III polyketide synthase (PKS) and subsequent modifying enzymes.
The biosynthesis of isoflavonoids involves several enzymes; These are:
Flavonoid 3',5'-hydroxylase (EC 1.14.14.81 was wrongly classified as EC 1.14.13.88 in the past) is an enzyme with systematic name flavanone,NADPH:oxygen oxidoreductase. This enzyme catalyses the following chemical reaction

Melitidin is a flavanone glycoside. Melitidin was discovered in bergamot orange juice and exhibits statin-like properties so the juice seems to have hypolipidemic activity.

Brutieridin is a flavanone glycoside. Brutieridin was discovered in bergamot orange juice and exhibits statin-like properties as well as an anticholesterolaemic effect. As a result, the juice seems to have hypolipidemic (lipid-lowering) activity.

Anthochlor pigments are a group of secondary plant metabolites and with carotenoids and some flavonoids produce yellow flower colour. Both, chalcones and aurones are known as anthochlor pigments. Anthochlor pigments serve as UV nectar guides in some plants. Important anthochlor pigments accumulating plants are from the genus Coreopsis, Snapdragon or Bidens ferulifolia.














