
Flavonoids are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.

Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
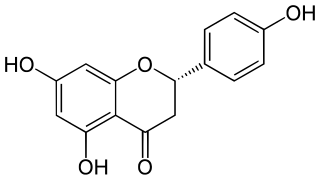
Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs.
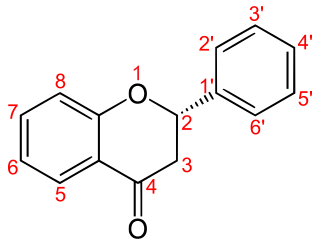
The flavanones, a type of flavonoids, are various aromatic, colorless ketones derived from flavone that often occur in plants as glycosides.

Gentisic acid is a dihydroxybenzoic acid. It is a derivative of benzoic acid and a minor (1%) product of the metabolic break down of aspirin, excreted by the kidneys.

Myb genes are part of a large gene family of transcription factors found in animals and plants. In humans, it includes Myb proto-oncogene like 1 and Myb-related protein B in addition to MYB proper. Members of the extended SANT/Myb family also include the SANT domain and other similar all-helical homeobox-like domains.
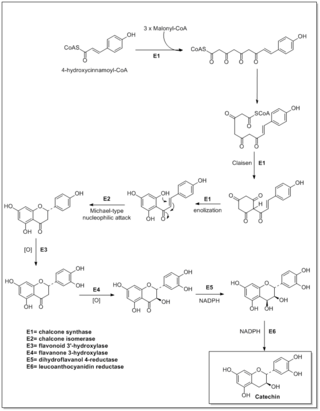
Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols.

The 3-Deoxyanthocyanidins and their glycosides are molecules with an anthocyanidins backbone lacking an hydroxyl group at position 3 on the C-ring. This nomenclature is the inverse of that which is commonly used in flavonoids, where the hydroxy-group is assumed absent if it is not specified, e. g. flavan-3-ol, flavan-4-ol, flavan-3,4-ol and flavonol.

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.

The flavans are benzopyran derivatives that use the 2-phenyl-3,4-dihydro-2H-chromene skeleton. They may be found in plants. These compounds include the flavan-3-ols, flavan-4-ols and flavan-3,4-diols (leucoanthocyanidin).

Afzelechin is a flavan-3-ol, a type of flavonoid. It can be found in Bergenia ligulata. It exists as at least 2 major epimers.

Phlobaphenes are reddish, alcohol-soluble and water-insoluble phenolic substances. They can be extracted from plants, or be the result from treatment of tannin extracts with mineral acids. The name phlobaphen come from the Greek roots φλoιὀς (phloios) meaning bark and βαφή (baphe) meaning dye.

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.
Menisciopsis penangiana is a fern species in the genus Menisciopsis. It has many synonyms, including Abacopteris penangiana and Pronephrium penangianum.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

Catechin-7-O-glucoside is a flavan-3-ol glycoside formed from catechin.

Iris tenuifolia is a beardless iris in the genus Iris, in the subgenus Limniris and in the series Tenuifoliae of the genus. It is a rhizomatous herbaceous perennial, from a wide region over central Asia, including Afghanistan, Pakistan, ; Kazakhstan, Uzbekistan and Mongolia and in China. It has long greyish-green leaves, short stem and pale violet, lilac, pale blue, or purple flowers.




















