
Elias James Corey is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.

Vanillin is an organic compound with the molecular formula C8H8O3. It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the extract of the vanilla bean. Synthetic vanillin is now used more often than natural vanilla extract as a flavoring in foods, beverages, and pharmaceuticals.
Dalbergia is a large genus of small to medium-size trees, shrubs and lianas in the pea family, Fabaceae, subfamily Faboideae. It was recently assigned to the informal monophyletic Dalbergia clade : the Dalbergieae. The genus has a wide distribution, native to the tropical regions of Central and South America, Africa, Madagascar and southern Asia.

Cinnamaldehyde is an organic compound with the formula() C6H5CH=CHCHO. Occurring naturally as predominantly the trans (E) isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus Cinnamomum. The essential oil of cinnamon bark is about 90% cinnamaldehyde. Cinnamaldehyde decomposes to styrene because of oxidation as a result of bad storage or transport conditions. Styrene especially forms in high humidity and high temperatures. This is the reason why cinnamon contains small amounts of styrene.

Piperonal, also known as heliotropin, is an organic compound which is commonly found in fragrances and flavors. The molecule is structurally related to other aromatic aldehydes such as benzaldehyde and vanillin.
The Bischler–Napieralski reaction is an intramolecular electrophilic aromatic substitution reaction that allows for the cyclization of β-arylethylamides or β-arylethylcarbamates. It was first discovered in 1893 by August Bischler and Bernard Napieralski, in affiliation with Basle Chemical Works and the University of Zurich. The reaction is most notably used in the synthesis of dihydroisoquinolines, which can be subsequently oxidized to isoquinolines.

Scutellaria is a genus of flowering plants in the mint family, Lamiaceae. They are known commonly as skullcaps. The generic name is derived from the Latin scutella, meaning "a small dish, tray or platter", or "little dish", referring to the shape of the calyx. The common name alludes to the resemblance of the same structure to "miniature medieval helmets". The genus has a subcosmopolitan distribution, with species occurring nearly worldwide, mainly in temperate regions.

Rosewood refers to any of a number of richly hued timbers, often brownish with darker veining, but found in many different hues and colours.
Neoflavonoids are a class of polyphenolic compounds. While flavonoids have the 2-phenylchromen-4-one backbone, neoflavonoids have the 4-phenylchromen backbone with no hydroxyl group substitution at position 2.

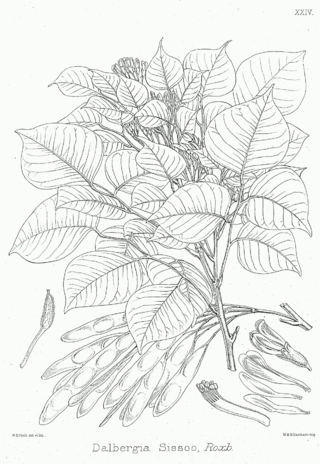
Dalbergia sissoo, known commonly as North Indian rosewood or shisham, is a fast-growing, hardy, deciduous rosewood tree native to the Indian subcontinent and southern Iran. D. sissoo is a large, crooked tree with long, leathery leaves and whitish or pink flowers.

Vanillic acid is a dihydroxybenzoic acid derivative used as a flavoring agent. It is an oxidized form of vanillin. It is also an intermediate in the production of vanillin from ferulic acid.

ortho-Vanillin (2-hydroxy-3-methoxybenzaldehyde) is an organic solid present in the extracts and essential oils of many plants. Its functional groups include aldehyde, ether and phenol. ortho-Vanillin, a compound of the formula C8H8O3, is distinctly different from its more prevalent isomer, meta-vanillin. The "ortho-" prefix refers to the position of the compound’s hydroxyl moiety, which is found in the para-position in vanillin.
The Danheiser annulation or Danheiser TMS-cyclopentene annulation is an organic reaction of an α,β-unsaturated ketone and a trialkylsilylallene in the presence of a Lewis Acid to give a trialkylsilylcyclopentene in a regiocontrolled annulation.

miR-134 is a family of microRNA precursors found in mammals, including humans. MicroRNAs are typically transcribed as ~70 nucleotide precursors and subsequently processed by the Dicer enzyme to give a ~22 nucleotide product. The excised region or, mature product, of the miR-134 precursor is the microRNA mir-134.
In molecular biology mir-339 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms. miR-339-5p expression was associated with overall survival in breast cancer.
In molecular biology mir-367 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.

Vanillylamine is a chemical compound that is an intermediate in the biosynthesis of capsaicin. Vanillylamine is produced from vanillin by the enzyme vanillin aminotransferase. It is then converted with 8-methyl-6-nonenoic acid into capsaicin by the enzyme capsaicin synthase.

The dalbergioids are an early-branching monophyletic clade of the flowering plant subfamily Faboideae or Papilionaceae. They are pantropical, particularly being found in the neotropics and sub-Saharan Africa. This clade is consistently resolved as monophyletic in molecular phylogenetic analyses. It is estimated to have arisen 55.3 ± 0.5 million years ago. A node-based definition for the dalbergioids is: "The least inclusive crown clade that contains Amorpha fruticosaL. 1753 and Dalbergia sissooRoxb. ex DC. 1825." Indehiscent pods may be a morphological synapomorphy for the clade.
In organic chemistry, the Davis oxidation or Davis' oxaziridine oxidation refers to oxidations involving the use of the Davis reagent or other similar oxaziridine reagents. This reaction mainly refers to the generation of α-hydroxy carbonyl compounds (acyloins) from ketones or esters. The reaction is carried out in a basic environment to generate the corresponding enolate from the ketone or ester. This reaction has been shown to work for amides.
In organic chemistry, the Lombardo methylenation is a name reaction that allows for the methylenation of carbonyl compounds with the use of Lombardo's reagent, which is a mix of zinc, dibromomethane, and titanium tetrachloride.












