
Lamium purpureum, known as red dead-nettle, purple dead-nettle, or purple archangel, is an annual herbaceous flowering plant native to Europe and Asia.
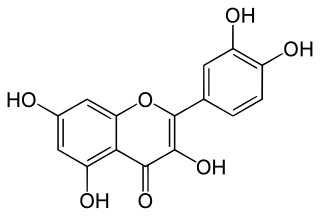
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.
The Ferrier rearrangement is an organic reaction that involves a nucleophilic substitution reaction combined with an allylic shift in a glycal. It was discovered by the carbohydrate chemist Robert J. Ferrier.
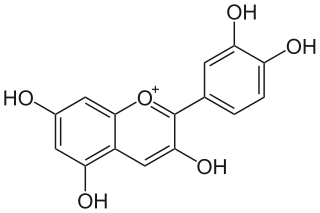
Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.
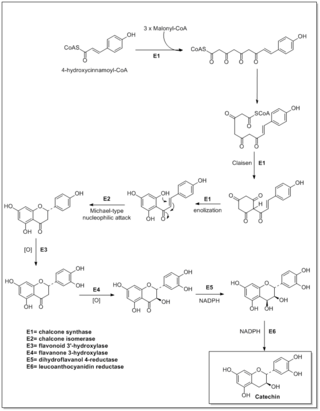
Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.
In enzymology, an isoflavone-7-O-beta-glucoside 6"-O-malonyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a flavonol-3-O-glucoside L-rhamnosyltransferase is an enzyme that catalyzes the chemical reaction

In enzymology, a flavonol 3-O-glucosyltransferase is an enzyme that catalyzes the chemical reaction

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Trifolin is a chemical compound. It is the kaempferol 3-galactoside. It can be found in Camptotheca acuminata, in Euphorbia condylocarpa or in Consolida oliveriana.

Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols. It is extracted from plants such as Siberian larch and milk thistle.

Aromadendrin is a flavanonol, a type of flavonoid. It can be found in the wood of Pinus sibirica.

Astilbin is a flavanonol, a type of flavonoid. Astilbin is the (2R-trans)-isomer; neoisoastilbin is the (2S-cis)-isomer and isoastilbin is the (2R-cis)-isomer.

Smitilbin is a flavanonol, a type of flavonoid. It is a rhamnoside that can be isolated in Smilax glabra.
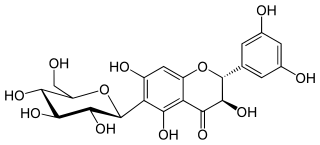
Xeractinol is a flavanonol, a type of flavonoid. It is a glucoside that can be found in the leaves of Paepalanthus argenteus (Eriocaulaceae).

Sarsasapogenin is a steroidal sapogenin, that is the aglycosidic portion of a plant saponin. It is named after sarsaparilla, a family of climbing plants found in subtropical regions. It was one of the first sapogenins to be identified, and the first spirostan steroid to be identified as such. The identification of the spirostan structure, with its ketone spiro acetal functionality, was fundamental in the development of the Marker degradation, which allowed the industrial production of progesterone and other sex hormones from plant steroids.
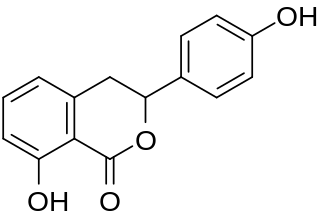
Hydrangenol is a dihydroisocoumarin. It can be found in Hydrangea macrophylla, as well as its 8-O-glucoside. (−)-Hydrangenol 4′-O-glucoside and (+)-hydrangenol 4′-O-glucoside can be found in Hydrangeae Dulcis Folium, the processed leaves of H. macrophylla var. thunbergii.

Smilax glabra, sarsaparilla, is a plant species in the genus Smilax. It is native to China, the Himalayas, and Indochina.

Kaempferol 7-O-glucoside is a flavonol glucoside. It can be found in Smilax china, and in the fern Asplenium rhizophyllum, and its hybrid descendants, as part of a complex with caffeic acid.

Smilax china is a climbing plant species in the genus Smilax. It is native to China, Korea, Taiwan, Japan, Philippines, Vietnam, Thailand, Myanmar, and India. It also known as china root, china-root, or chinaroot, as is the related Smilax glabra.

















