
Neurotoxins are toxins that are destructive to nerve tissue. Neurotoxins are an extensive class of exogenous chemical neurological insults that can adversely affect function in both developing and mature nervous tissue. The term can also be used to classify endogenous compounds, which, when abnormally contacted, can prove neurologically toxic. Though neurotoxins are often neurologically destructive, their ability to specifically target neural components is important in the study of nervous systems. Common examples of neurotoxins include lead, ethanol, glutamate, nitric oxide, botulinum toxin, tetanus toxin, and tetrodotoxin. Some substances such as nitric oxide and glutamate are in fact essential for proper function of the body and only exert neurotoxic effects at excessive concentrations.

Maclura tinctoria, known as old fustic and dyer's mulberry, is a medium to large tree of the Neotropics, from Mexico to Argentina. It produces a yellow dye called fustic primarily known for coloring khaki fabric for U.S. military apparel during World War I. This dye contains the flavonoid morin. It is dioecious, so both male and female plants are needed to set seed.

Cotinus coggygria, syn. Rhus cotinus, the European smoketree, Eurasian smoketree, smoke tree, smoke bush, Venetian sumach, or dyer's sumach, is a Eurasian species of flowering plant in the family Anacardiaceae.
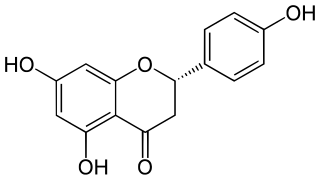
Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs.
Neuroprotection refers to the relative preservation of neuronal structure and/or function. In the case of an ongoing insult the relative preservation of neuronal integrity implies a reduction in the rate of neuronal loss over time, which can be expressed as a differential equation. It is a widely explored treatment option for many central nervous system (CNS) disorders including neurodegenerative diseases, stroke, traumatic brain injury, spinal cord injury, and acute management of neurotoxin consumption. Neuroprotection aims to prevent or slow disease progression and secondary injuries by halting or at least slowing the loss of neurons. Despite differences in symptoms or injuries associated with CNS disorders, many of the mechanisms behind neurodegeneration are the same. Common mechanisms of neuronal injury include decreased delivery of oxygen and glucose to the brain, energy failure, increased levels in oxidative stress, mitochondrial dysfunction, excitotoxicity, inflammatory changes, iron accumulation, and protein aggregation. Of these mechanisms, neuroprotective treatments often target oxidative stress and excitotoxicity—both of which are highly associated with CNS disorders. Not only can oxidative stress and excitotoxicity trigger neuron cell death but when combined they have synergistic effects that cause even more degradation than on their own. Thus limiting excitotoxicity and oxidative stress is a very important aspect of neuroprotection. Common neuroprotective treatments are glutamate antagonists and antioxidants, which aim to limit excitotoxicity and oxidative stress respectively.

Diallyl disulfide is an organosulfur compound derived from garlic and a few other genus Allium plants. Along with diallyl trisulfide and diallyl tetrasulfide, it is one of the principal components of the distilled oil of garlic. It is a yellowish liquid which is insoluble in water and has a strong garlic odor. It is produced during the decomposition of allicin, which is released upon crushing garlic and other plants of the family Alliaceae. Diallyl disulfide has many of the health benefits of garlic, but it is also an allergen causing garlic allergy. Highly diluted, it is used as a flavoring in food. It decomposes in the human body into other compounds such as allyl methyl sulfide.
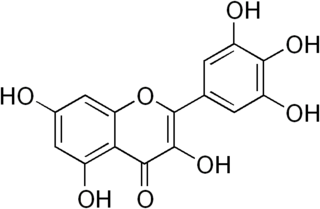
Myricetin is a member of the flavonoid class of polyphenolic compounds, with antioxidant properties. Common dietary sources include vegetables, fruits, nuts, berries, tea, and red wine. Myricetin is structurally similar to fisetin, luteolin, and quercetin and is reported to have many of the same functions as these other members of the flavonol class of flavonoids. Reported average intake of myricetin per day varies depending on diet, but has been shown in the Netherlands to average 23 mg/day.

Genistein (C15H10O5) is a naturally occurring compound that structurally belongs to a class of compounds known as isoflavones. It is described as an angiogenesis inhibitor and a phytoestrogen.

RE1-Silencing Transcription factor (REST), also known as Neuron-Restrictive Silencer Factor (NRSF), is a protein which in humans is encoded by the REST gene, and acts as a transcriptional repressor. REST is expressly involved in the repression of neural genes in non-neuronal cells. Many genetic disorders have been tied to alterations in the REST expression pattern, including colon and small-cell lung carcinomas found with truncated versions of REST. In addition to these cancers, defects in REST have also been attributed a role in Huntington Disease, neuroblastomas, and the effects of epileptic seizures and ischemia.

Brain mitochondrial carrier protein 1 is a protein that in humans is encoded by the SLC25A14 gene.

Quinolinic acid, also known as pyridine-2,3-dicarboxylic acid, is a dicarboxylic acid with a pyridine backbone. It is a colorless solid. It is the biosynthetic precursor to niacin.

Cornus officinalis, the Japanese cornel or Japanese cornelian cherry, is a species of flowering plant in the dogwood family Cornaceae. Despite its name, it is native to China and Korea as well as Japan. It is not to be confused with C. mas, which is also known as the Cornelian cherry. It is not closely related to the true cherries of the genus Prunus.
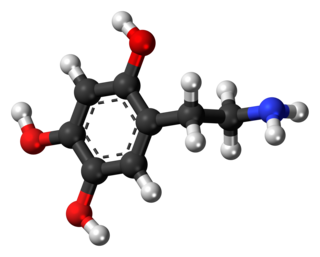
Oxidopamine, also known as 6-hydroxydopamine (6-OHDA) or 2,4,5-trihydroxyphenethylamine, is a neurotoxic synthetic organic compound used by researchers to selectively destroy dopaminergic and noradrenergic neurons in the brain.

Tricetin is a flavone, a type of flavonoid. It is a rare aglycone found in the pollen of members of the Myrtaceae, subfamily Leptospermoideae, such as Eucalyptus globulus. This compound shows anticancer effects on human breast adenocarcinoma MCF-7 cells. Moreover. a potent anti-inflammatory effect of tricetin has also been demonstrated in a model of acute pancreatitis. This observation was explained by the compound's radical scavenging effects, its inhibitory effect on the DNA damage sensor enzyme poly (ADP-ribose) polymerase-1 (PARP1) and PARP1-mediated cell death and suppression of inflammatory gene expression.

Scutellarin is a flavone, a type of phenolic chemical compound. It can be found in the Asian "barbed skullcap" Scutellaria barbata and the north American plant S. lateriflora both of which have been used in traditional medicine. The compound is found only in trace amounts in the "Chinese skullcap" Scutellaria baicalensis, another plant used in traditional Chinese medicine.

Fisetin (7,3′,4′-flavon-3-ol) is a plant flavonol from the flavonoid group of polyphenols. It can be found in many plants, where it serves as a yellow/ochre colouring agent. It is also found in many fruits and vegetables, such as strawberries, apples, persimmons, onions and cucumbers. Its chemical formula was first described by Austrian chemist Josef Herzig in 1891.
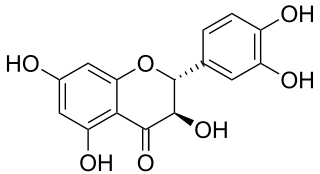
Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols.

Ampelopsin, also known as dihydromyricetin and DHM, when purported as an effective ingredient in supplements and other tonics, is a flavanonol, a type of flavonoid. It is extracted from the Japanese raisin tree and found in Ampelopsis species japonica, megalophylla, and grossedentata; Cercidiphyllum japonicum; Hovenia dulcis; Rhododendron cinnabarinum; some Pinus species; and some Cedrus species, as well as in Salix sachalinensis.
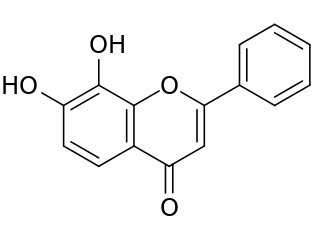
Tropoflavin, also known as 7,8-dihydroxyflavone, is a naturally occurring flavone found in Godmania aesculifolia, Tridax procumbens, and primula tree leaves. It has been found to act as a potent and selective small-molecule agonist of the tropomyosin receptor kinase B (TrkB), the main signaling receptor of the neurotrophin brain-derived neurotrophic factor (BDNF). Tropoflavin is both orally bioavailable and able to penetrate the blood–brain barrier. A prodrug of tropoflavin with greatly improved potency and pharmacokinetics, R13, is under development for the treatment of Alzheimer's disease.
Valina L. Dawson is an American neuroscientist who is the director of the Programs in Neuroregeneration and Stem Cells at the Institute for Cell Engineering at the Johns Hopkins University School of Medicine. She has joint appointments in the Department of Neurology, Neuroscience and Physiology. She is a member of the Graduate Program in Cellular and Molecular Medicine and Biochemistry, Cellular and Molecular Biology.


















