Artemis is a protein that in humans is encoded by the DCLRE1C (DNA cross-link repair 1C) gene. [5] [6]
Artemis is a protein that in humans is encoded by the DCLRE1C (DNA cross-link repair 1C) gene. [5] [6]
Artemis is a nuclear protein that is involved in V(D)J recombination and DNA repair. The protein has endonuclease activity on 5' and 3' overhangs and hairpins when complexed with PRKDC. [7]
Artemis plays an essential role in V(D)J recombination, the process by which B cell antibody genes and T cell receptor genes are assembled from individual V (variable), D (diversity), and J (joining) segments. [8] For example, in joining a V segment to a D segment, the RAG (recombination activating gene) nuclease cuts both DNA strands adjacent to a V segment and adjacent to a D segment. The intervening DNA between the V and D segments is ligated to form a circular DNA molecule that is lost from the chromosome. [9] At each of the two remaining ends, called the coding ends, the two strands of DNA are joined to form a hairpin structure. Artemis nuclease, in a complex with the DNA-dependent protein kinase (DNA‑PK), binds to these DNA ends and makes a single cut near the tip of the hairpin. [10] The exposed 3' termini are subject to deletion and addition of nucleotides by a variety of exonucleases and DNA polymerases, before the V and D segments are ligated to restore the integrity of the chromosome. The exact site of cleavage of the hairpin by Artemis is variable, and this variability, combined with random nucleotide deletion and addition, confers extreme diversity upon the resulting antibody and T-cell receptor genes, thus allowing the immune system to mount an immune response to virtually any foreign antigen. [11] In Artemis-deficient individuals, V(D)J recombination is blocked because the hairpin ends cannot be opened, and so no mature B or T cells are produced, a condition known as severe combined immune deficiency (SCID). [10] Artemis was first identified as the gene defective in a subset of SCID patients that were unusually sensitive to radiation.
Cells deficient in Artemis are more sensitive than normal cells to X‑rays [5] and to chemical agents that induce double-strand breaks (DSBs), [12] and they show a higher incidence of chromosome breaks following irradiation. [13] Direct measurement of DSBs by pulsed-field electrophoresis indicates that in Artemis-deficient cells 75-90% of DSBs are repaired rapidly, just as in normal cells. However, the remaining 10-20% of DSBs that are repaired more slowly (2-24 hr) in normal cells, are not repaired at all in Artemis-deficient cells. [14] Repair of these presumably difficult-to-rejoin breaks also requires several other proteins, including the Mre11/Rad50/NBS1 complex, the ataxia telangiectasia mutated ATM kinase, and 53BP1. Because Artemis can remove damaged ends from DNA, [12] it has been proposed that these DSBs are those whose damaged ends require trimming by Artemis. However, evidence that both ATM and Artemis are specifically required for repair of DSBs in heterochromatin, [15] [16] has called this interpretation into question.
Artemis functions in the repair of DNA double-strand breaks that arise by induced oxidative reactions, or by endogenous reactions. [17] Such DNA repair occurs in heterochromatin as well as in euchromatin.
Mutations in this gene cause Athabascan-type severe combined immunodeficiency (SCIDA). [18]

Non-homologous end joining (NHEJ) is a pathway that repairs double-strand breaks in DNA. It is called "non-homologous" because the break ends are directly ligated without the need for a homologous template, in contrast to homology directed repair (HDR), which requires a homologous sequence to guide repair. NHEJ is active in both non-dividing and proliferating cells, while HDR is not readily accessible in non-dividing cells. The term "non-homologous end joining" was coined in 1996 by Moore and Haber.

ATM serine/threonine kinase or Ataxia-telangiectasia mutated, symbol ATM, is a serine/threonine protein kinase that is recruited and activated by DNA double-strand breaks, oxidative stress, topoisomerase cleavage complexes, splicing intermediates, R-loops and in some cases by single-strand DNA breaks. It phosphorylates several key proteins that initiate activation of the DNA damage checkpoint, leading to cell cycle arrest, DNA repair or apoptosis. Several of these targets, including p53, CHK2, BRCA1, NBS1 and H2AX are tumor suppressors.
V(D)J recombination is the mechanism of somatic recombination that occurs only in developing lymphocytes during the early stages of T and B cell maturation. It results in the highly diverse repertoire of antibodies/immunoglobulins and T cell receptors (TCRs) found in B cells and T cells, respectively. The process is a defining feature of the adaptive immune system.

Recombination activating gene 2protein is a lymphocyte-specific protein encoded by the RAG2 gene on human chromosome 11. Together with the RAG1 protein, RAG2 forms a V(D)J recombinase, a protein complex required for the process of V(D)J recombination during which the variable regions of immunoglobulin and T cell receptor genes are assembled in developing B and T lymphocytes. Therefore, RAG2 is essential for the generation of mature B and T lymphocytes.

Serine/threonine-protein kinase ATR, also known as ataxia telangiectasia and Rad3-related protein (ATR) or FRAP-related protein 1 (FRP1), is an enzyme that, in humans, is encoded by the ATR gene. It is a large kinase of about 301.66 kDa. ATR belongs to the phosphatidylinositol 3-kinase-related kinase protein family. ATR is activated in response to single strand breaks, and works with ATM to ensure genome integrity.

DNA repair protein XRCC4 (hXRCC4) also known as X-ray repair cross-complementing protein 4 is a protein that in humans is encoded by the XRCC4 gene. XRCC4 is also expressed in many other animals, fungi and plants. hXRCC4 is one of several core proteins involved in the non-homologous end joining (NHEJ) pathway to repair DNA double strand breaks (DSBs).

Nibrin, also known as NBN or NBS1, is a protein which in humans is encoded by the NBN gene.

DNA-dependent protein kinase catalytic subunit, also known as DNA-PKcs, is an enzyme that plays a crucial role in repairing DNA double-strand breaks and has a number of other DNA housekeeping functions. In humans it is encoded by the gene designated as PRKDC or XRCC7. DNA-PKcs belongs to the phosphatidylinositol 3-kinase-related kinase protein family. The DNA-Pkcs protein is a serine/threonine protein kinase consisting of a single polypeptide chain of 4,128 amino acids.

Bloom syndrome protein is a protein that in humans is encoded by the BLM gene and is not expressed in Bloom syndrome.

Exonuclease 1 is an enzyme that in humans is encoded by the EXO1 gene.
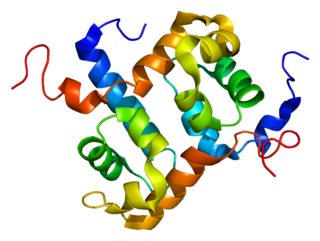
ERCC4 is a protein designated as DNA repair endonuclease XPF that in humans is encoded by the ERCC4 gene. Together with ERCC1, ERCC4 forms the ERCC1-XPF enzyme complex that participates in DNA repair and DNA recombination.
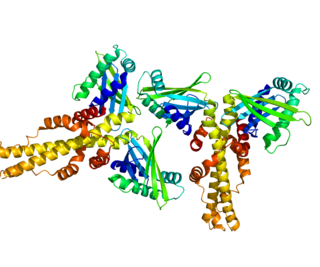
Non-homologous end-joining factor 1 (NHEJ1), also known as Cernunnos or XRCC4-like factor (XLF), is a protein that in humans is encoded by the NHEJ1 gene. XLF was originally discovered as the protein mutated in five patients with growth retardation, microcephaly, and immunodeficiency. The protein is required for the non-homologous end joining (NHEJ) pathway of DNA repair. Patients with XLF mutations also have immunodeficiency due to a defect in V(D)J recombination, which uses NHEJ to generate diversity in the antibody repertoire of the immune system. XLF interacts with DNA ligase IV and XRCC4 and is thought to be involved in the end-bridging or ligation steps of NHEJ. The yeast homolog of XLF is Nej1.

Junctional diversity describes the DNA sequence variations introduced by the improper joining of gene segments during the process of V(D)J recombination. This process of V(D)J recombination has vital roles for the vertebrate immune system, as it is able to generate a huge repertoire of different T-cell receptor (TCR) and immunoglobulin molecules required for pathogen antigen recognition by T-cells and B cells, respectively.

The meiotic recombination checkpoint monitors meiotic recombination during meiosis, and blocks the entry into metaphase I if recombination is not efficiently processed.

LIG4 syndrome is an extremely rare condition caused by mutations in the DNA Ligase IV (LIG4) gene. Some mutations in this gene are associated with a resistance against multiple myeloma and Severe Combined Immunodeficiency. Severity of symptoms depends on the degree of reduced enzymatic activity of Ligase IV or gene expression. Ligase IV is a critical component of the non-homologous end joining (NHEJ) mechanism that repairs DNA double-strand breaks. It is employed in repairing DNA double-strand breaks caused by reactive oxygen species produced by normal metabolism, or by DNA damaging agents such as ionizing radiation. NHEJ is also used to repair the DNA double-strand break intermediates that occur in the production of T and B lymphocyte receptors.
The Artemis complex is a protein complex that functions in V(D)J recombination, the somatic recombination process which generates diversity in T cell receptors and immunoglobulins. Mutations in the Artemis complex results in hypersensitivity to DNA double-strand break-inducing agents, such as radiation; and so people with mutations in the Artemis complex may develop radiosensitive severe combined immune deficiency (RS-SCID).

Synthesis-dependent strand annealing (SDSA) is a major mechanism of homology-directed repair of DNA double-strand breaks (DSBs). Although many of the features of SDSA were first suggested in 1976, the double-Holliday junction model proposed in 1983 was favored by many researchers. In 1994, studies of double-strand gap repair in Drosophila were found to be incompatible with the double-Holliday junction model, leading researchers to propose a model they called synthesis-dependent strand annealing. Subsequent studies of meiotic recombination in S. cerevisiae found that non-crossover products appear earlier than double-Holliday junctions or crossover products, challenging the previous notion that both crossover and non-crossover products are produced by double-Holliday junctions and leading the authors to propose that non-crossover products are generated through SDSA.
Penelope "Penny" Jeggo is a noted British molecular biologist, best known for her work in understanding damage to DNA. She is also known for her work with DNA gene mutations. Her interest in DNA damage has inspired her to research radiation biology and radiation therapy and how radiation affects DNA. Jeggo has more than 170 publications that pertain to DNA damage, radiation, and cancer research and has received 3 top science awards/medals for her research. Jeggo has also been a member of several organizations that pertain to radiation biology; these organizations include Committee on Medical Aspects of Radiation in the Environment (COMARE), National Institute for Radiation Science laboratory researcher, and the Multidisciplinary European Low Dose Initiative (MELODI). Jeggo is a member of these organizations, and she is also an editor for several publication journals that are related to cancer and radiation biology. Jeggo is very passionate about her research and in an interview with Fiona Watt claimed that “Although my results contributed only the tiniest smidgeon to scientific knowledge, I gained immense satisfaction from it”.

Immunodeficiency 26 is a rare genetic syndrome. It is characterised by absent circulating B and T cells and normal natural killer cells.

A double-strand break repair model refers to the various models of pathways that cells undertake to repair double strand-breaks (DSB). DSB repair is an important cellular process, as the accumulation of unrepaired DSB could lead to chromosomal rearrangements, tumorigenesis or even cell death. In human cells, there are two main DSB repair mechanisms: Homologous recombination (HR) and non-homologous end joining (NHEJ). HR relies on undamaged template DNA as reference to repair the DSB, resulting in the restoration of the original sequence. NHEJ modifies and ligates the damaged ends regardless of homology. In terms of DSB repair pathway choice, most mammalian cells appear to favor NHEJ rather than HR. This is because the employment of HR may lead to gene deletion or amplification in cells which contains repetitive sequences. In terms of repair models in the cell cycle, HR is only possible during the S and G2 phases, while NHEJ can occur throughout whole process. These repair pathways are all regulated by the overarching DNA damage response mechanism. Besides HR and NHEJ, there are also other repair models which exists in cells. Some are categorized under HR, such as synthesis-dependent strain annealing, break-induced replication, and single-strand annealing; while others are an entirely alternate repair model, namely, the pathway microhomology-mediated end joining (MMEJ).