Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Food are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.

Nicotine is a naturally produced alkaloid in the nightshade family of plants and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve withdrawal symptoms. Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits where it acts as a receptor antagonist.

Lipoic acid (LA), also known as α-lipoic acid, alpha-lipoic acid (ALA) and thioctic acid, is an organosulfur compound derived from caprylic acid (octanoic acid). ALA is made in animals normally, and is essential for aerobic metabolism. It is also manufactured and is available as a dietary supplement in some countries where it is marketed as an antioxidant, and is available as a pharmaceutical drug in other countries. Lipoate is the conjugate base of lipoic acid, and the most prevalent form of LA under physiological conditions. Only the (R)-(+)-enantiomer (RLA) exists in nature and is essential for aerobic metabolism because RLA is an essential cofactor of many enzyme complexes.
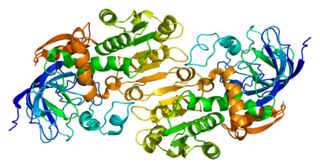
Alcohol dehydrogenases (ADH) (EC 1.1.1.1) are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to NADH. In humans and many other animals, they serve to break down alcohols that are otherwise toxic, and they also participate in the generation of useful aldehyde, ketone, or alcohol groups during the biosynthesis of various metabolites. In yeast, plants, and many bacteria, some alcohol dehydrogenases catalyze the opposite reaction as part of fermentation to ensure a constant supply of NAD+.

Polyphenols are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Doxepin is a medication belonging to the tricyclic antidepressant (TCA) class of drugs used to treat major depressive disorder, anxiety disorders, chronic hives, and insomnia. For hives it is a less preferred alternative to antihistamines. It has a mild to moderate benefit for sleeping problems. It is used as a cream for itchiness due to atopic dermatitis or lichen simplex chronicus.

Cyclophosphamide (CP), also known as cytophosphane among other names, is a medication used as chemotherapy and to suppress the immune system. As chemotherapy it is used to treat lymphoma, multiple myeloma, leukemia, ovarian cancer, breast cancer, small cell lung cancer, neuroblastoma, and sarcoma. As an immune suppressor it is used in nephrotic syndrome, granulomatosis with polyangiitis, and following organ transplant, among other conditions. It is taken by mouth or injection into a vein.

Peltigera is a genus of approximately 100 species of foliose lichens in the family Peltigeraceae. Commonly known as the dog or pelt lichens, species of Peltigera are often terricolous, but can also occur on moss, trees, rocks, and many other substrates in many parts of the world.

Punctelia is a genus of foliose lichens belonging to the large family Parmeliaceae. The genus, which contains about 50 species, was segregated from genus Parmelia in 1982. Characteristics that define Punctelia include the presence of hook-like to thread-like conidia, simple rhizines, and point-like pseudocyphellae. It is this last feature that is alluded to in the vernacular names speckled shield lichens or speckleback lichens.

Chemical defense is a strategy employed by many organisms to avoid consumption by producing toxic or repellent metabolites or chemical warnings which incite defensive behavioral changes. The production of defensive chemicals occurs in plants, fungi, and bacteria, as well as invertebrate and vertebrate animals. The class of chemicals produced by organisms that are considered defensive may be considered in a strict sense to only apply to those aiding an organism in escaping herbivory or predation. However, the distinction between types of chemical interaction is subjective and defensive chemicals may also be considered to protect against reduced fitness by pests, parasites, and competitors. Repellent rather than toxic metabolites are allomones, a sub category signaling metabolites known as semiochemicals. Many chemicals used for defensive purposes are secondary metabolites derived from primary metabolites which serve a physiological purpose in the organism. Secondary metabolites produced by plants are consumed and sequestered by a variety of arthropods and, in turn, toxins found in some amphibians, snakes, and even birds can be traced back to arthropod prey. There are a variety of special cases for considering mammalian antipredatory adaptations as chemical defenses as well.
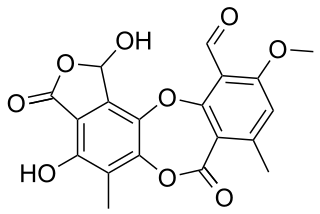
Stictic acid is an aromatic organic compound, a product of secondary metabolism in some species of lichens.

Botryosphaeran is an exopolysaccharide (EPS) produced by the ascomyceteous fungus Botryosphaeria rhodina. Characterization of the chemical structure of botryosphaeran showed this EPS to be a (1→3)(1→6)-β-D-glucan. This particular β-glucan can be produced by several strains of Botryosphaeria rhodina that include: MAMB-05, DABAC-P82, and RCYU 30101. Botryosphaeran exhibits interesting rheological properties and novel biological functions including hypoglycaemia, hypocholesterolaemia, anti-atheroslerosis and anti-cancer activity, with potential commercial applications. Three cosmetic products formulated with botryosphaeran have been developed to promote skin health and treat skin conditions for future intended commercialization purposes.

Lecanoric acid is a chemical produced by several species of lichen. Lecanoric acid is classified as a polyphenol and a didepside and it functions as an antioxidant. The acid is named after the lichen Lecanora. The acid has also been isolated from Usnea subvacata, Parmotrema stuppuem, Parmotrema tinctorum,Parmotrema grayana, Xanthoparmelia arida and Xanthoparmelia lecanorica. A related compound, 5-chlorolecanoric acid, is found in some species of Punctelia.

Stereocaulon ramulosum, commonly known as snow lichen, is a terricolous fruticose lichen belonging to the family Stereocaulaceae. It has cosmopolitan distribution. In the Australasian region, it is common in eastern Australia, New Zealand and has also been recorded at Lord Howe Island and Macquarie Island.

Punctelia borreri is a species of foliose lichen in the family Parmeliaceae. It is a common and widely distributed species, occurring in tropical, subtropical, and temperate regions of Africa, Asia, Europe, North America, Oceania, and South America. The lichen typically grows on bark of deciduous trees, and less commonly on rock. Some European countries have reported increases in the geographic range or regional frequency of the lichen in recent decades, attributed alternatively to a reduction of atmospheric sulphur dioxide levels or an increase in temperatures resulting from climate change.

Salazinic acid is a depsidone with a lactone ring. It is found in some lichens, and is especially prevalent in Parmotrema and Bulbothrix, where its presence or absence is often used to help classify species in those genera.

Lichexanthone is an organic compound in the structural class of chemicals known as xanthones. Lichexanthone was first isolated and identified by Japanese chemists from a species of leafy lichen in the 1940s. The compound is known to occur in many lichens, and it is important in the taxonomy of species in several genera, such as Pertusaria and Pyxine. More than a dozen lichen species have a variation of the word lichexanthone incorporated as part of their binomial name. The presence of lichexanthone in lichens causes them to fluoresce a greenish-yellow colour under long-wavelength UV light; this feature is used to help identify some species. Lichexanthone is also found in several plants, and some species of fungi that do not form lichens.

Pulchrocladia retipora, commonly known as the coral lichen, is a species of fruticose lichen in the family Cladoniaceae. Found predominantly in Australasia, its habitats range from the Australian Capital Territory to New Zealand's North and South Islands, and even the Pacific region of New Caledonia, where it grows in coastal and alpine heathlands. The lichen features coral-like branches and subbranches with numerous intricate, netlike perforations. It is known by multiple names, with some sources referring to it by its synonym Cladia retipora, or the common name lace lichen.
Lichen products, also known as lichen substances, are organic compounds produced by a lichen. Specifically, they are secondary metabolites. Lichen products are represented in several different chemical classes, including terpenoids, orcinol derivatives, chromones, xanthones, depsides, and depsidones. Over 800 lichen products of known chemical structure have been reported in the scientific literature, and most of these compound are exclusively found in lichens. Examples of lichen products include usnic acid, atranorin, lichexanthone, salazinic acid, and isolichenan, an α-glucan. Many lichen products have biological activity, and research into these effects is ongoing.

Barbatic acid is an organic compound that is made by some lichens. It is in the structural class known as depsides. It is particularly common in the genera Usnea and Cladonia.

















