Contents
 | |
| Names | |
|---|---|
| IUPAC name 12,14a,3-(Epoxymethyno)-2H-1-benzoxacyclododecin-2,4,8(5H,10aH)-trione, 6,7,11,12,13,14-hexahydro-11-hydroxy-5,7,13-trimethyl-, (5R,7S,9E,10aR,11R,12R,13R,14aR) | |
| Other names Atrop-abyssomicin C | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| |
| |
| Properties | |
| C19H22O6 | |
| Molar mass | 346.38 g/mol |
| Density | 1.34±0.1 g/cm3 (Predicted) |
| Melting point | 180 °C (decomp) |
| Boiling point | 597.5±50.0 °C (Predicted) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
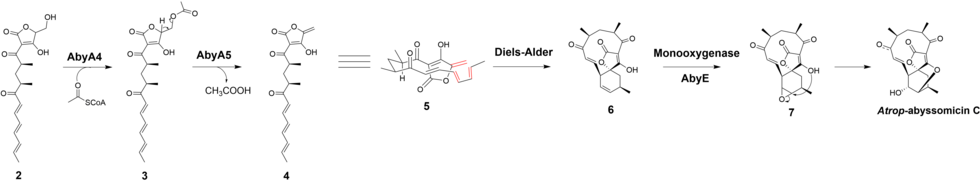
Atrop-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin. [1] In 2006, the Nicolaou group discovered atrop-abyssomicin C while working on the total synthesis of abyssomicin C. [2] Then in 2007, Süssmuth and co-workers isolated atrop-abyssomicin C from Verrucosispora maris AB-18-032, a marine actinomycete found in sediment of the Japanese sea. They found that atrop-abyssomicin C was the major metabolite produced by this strain, while abyssomicin C was a minor product. The molecule displays antibacterial activity by inhibiting the enzyme PabB (4-amino-4-deoxychorismate synthase), thereby depleting the biosynthesis of p -aminobenzoate. [3] [4]