 | |
| Names | |
|---|---|
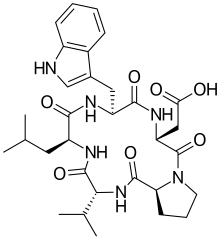
| IUPAC name 2-[(3R,6R,9S,12R,15S)-6-(1H-indol-3-ylmethyl)-9-(2-methylpropyl)-2,5,8,11,14-pentaoxo-12-propan-2-yl-1,4,7,10,13-pentazabicyclo[13.3.0]octadecan-3-yl]acetic acid | |
| Other names Cyclo(D-trp-D-asp-L-pro-D-val-L-leu) | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C31H42N6O7 | |
| Molar mass | 610.712 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
BQ-123, also known as cyclo(-D-Trp-D-Asp-Pro-D-Val-Leu-), is a cyclic pentapeptide that was first isolated from a fermentation broth of Streptomyces misakiensis in 1991. [2] NMR studies indicate that the polypeptide backbone consists of a type II beta turn and an inverse gamma turn. [3] [4] The side-chains adopt different orientations depending on the solvent used. [5] [6] The proline carbonyl oxygen atom located at the onset of a beta turn is a sodium ion binding site. [7] It has a high affinity for sodium ions and can coordinate up to three of them. [8] Studies have shown that BQ123 is effective in reversing Ischemia-induced acute renal failure, and it has been suggested that this might be because BQ123 increases reabsorption of sodium ions in the proximal tubule cells. [9] [10] [11] [12] [13]
BQ-123 is a selective ETA endothelin receptor antagonist. [1] [14] As such, it is used as a biochemical tool in the study of endothelin receptor function. BQ-123 works as an ET-1 antagonist by reversing already established contractions to ET-1. This indicates that BQ-123 can work as an antagonist to remove ET-1 from its receptor (ETA). [15]