Armillaria mellea, commonly known as honey fungus, is a basidiomycete fungus in the genus Armillaria. It is a plant pathogen and part of a cryptic species complex of closely related and morphologically similar species. It causes Armillaria root rot in many plant species and produces mushrooms around the base of trees it has infected. The symptoms of infection appear in the crowns of infected trees as discoloured foliage, reduced growth, dieback of the branches and death. The mushrooms are edible but some people may be intolerant to them. This species is capable of producing light via bioluminescence in its mycelium.

Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of cardiovascular disease. Its use is recommended together with lifestyle changes. It is taken by mouth.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

Melanogaster is a genus of fungus that resemble truffles, and are often mistaken for them. However, they do not have the characteristic aroma and value of truffles, although some have been used culinarily. None are known to be poisonous. The genus contains 25 species that collectively have a widespread distribution.

Neocarzinostatin (NCS) is a macromolecular chromoprotein enediyne antitumor antibiotic secreted by Streptomyces macromomyceticus.
Polyketide synthases (PKSs) are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages. The biosyntheses of polyketides share striking similarities with fatty acid biosynthesis.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of streptomyces. In contrast, only one known non-wild type species, streptomyces peucetius subspecies cesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.
In enzymology, an erythronolide synthase is an enzyme that catalyzes the chemical reaction

Zaragozic acids are a family of natural products produced by fungi. The first characterized zaragozic acids, A, B, and C were isolated from an unidentified sterile fungal culture, Sporormiella intermedia, and L. elatius, respectively. just outside the European city Zaragoza, Spain on the Jalón river. This family of natural products possesses a unique 4,8-dioxabicyclo[3.2.1]octane core, and vary in their 1-alkyl and their 6-acyl side chains.
Streptogramin A is a group of antibiotics within the larger family of antibiotics known as streptogramins. They are synthesized by the bacteria Streptomyces virginiae. The streptogramin family of antibiotics consists of two distinct groups: group A antibiotics contain a 23-membered unsaturated ring with lactone and peptide bonds while group B antibiotics are depsipeptides. While structurally different, these two groups of antibiotics act synergistically, providing greater antibiotic activity than the combined activity of the separate components. These antibiotics have until recently been commercially manufactured as feed additives in agriculture, although today there is increased interest in their ability to combat antibiotic-resistant bacteria, particularly vancomycin-resistant bacteria.
Chaetomium cupreum is a fungus in the family, Chaetomiaceae. It is able to decay in manufactured cellulosic materials, and is known to antagonize a wide range of soil microorganisms. This species is component of the biocontrol agent, Ketomium, a commercial biofungicide. It has also been investigated for use in the production of natural dyes. Chaetomium cupreum is mesophilic and known to occur in harsh environments and can rapidly colonize organic substrates in soil. Laboratory cultures of C. cupreum can be propagated on a range of common growth media including potato dextrose at ambient or higher than ambient temperature producing cottony white colonies with a reddish reverse.

Mycena aurantiomarginata, commonly known as the golden-edge bonnet, is a species of agaric fungus in the family Mycenaceae. First formally described in 1803, it was given its current name in 1872. Widely distributed, it is common in Europe and North America, and has also been collected in North Africa, Central America, and Japan. The fungus is saprobic, and produces fruit bodies (mushrooms) that grow on the floor of coniferous forests. The mushrooms have a bell-shaped to conical cap up to 2 cm (0.8 in) in diameter, set atop a slender stipe up to 6 cm (2.4 in) long with yellow to orange hairs at the base. The fungus is named after its characteristic bright orange gill edges. A microscopic characteristic is the club-shaped cystidia that are covered with numerous spiky projections, resembling a mace. The edibility of the mushroom has not been determined. M. aurantiomarginata can be distinguished from similar Mycena species by differences in size, color, and substrate. A 2010 publication reported the discovery and characterization of a novel pigment named mycenaaurin A, isolated from the mushroom. The pigment is responsible for its color, and it has antibiotic activity that may function to prevent certain bacteria from growing on the mushroom.

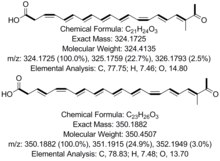
Codinaeopsin is an antimalarial isolated from a fungal isolate found in white yemeri trees (Vochysia guatemalensis) in Costa Rica. It is reported to have bioactivity against Plasmodium falciparum with an IC50 = 2.3 μg/mL (4.7 μM). Pure codinaeopsin was reported to be isolated with a total yield of 18 mg/mL from cultured fungus. The biosynthesis of codinaeopsin involves a polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) hybrid.
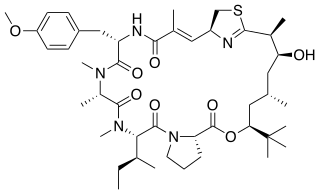
Apratoxin A - is a cyanobacterial secondary metabolite, known as a potent cytotoxic marine natural product. It is a derivative of the Apratoxin family of cytotoxins. The mixed peptide-polyketide natural product comes from a polyketide synthase/non-ribosomal peptide synthase pathway (PKS/NRPS). This cytotoxin is known for inducing G1-phase cell cycle arrest and apoptosis. This natural product's activity has made it a popular target for developing anticancer derivatives.
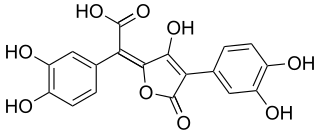
Variegatic acid is an orange pigment found in some mushrooms. It is responsible for the bluing reaction seen in many bolete mushrooms when they are injured. When mushroom tissue containing variegatic acid is exposed to air, the chemical is enzymatically oxidized to blue quinone methide anions, specifically chinonmethid anions. It is derived from xerocomic acid, which is preceded by atromentic acid and atromentin, and its genetic basis is unknown. In its oxidized form is variegatorubin, similar to xerocomorubin.

Ketoacyl synthases (KSs) catalyze the condensation reaction of acyl-CoA or acyl-acyl ACP with malonyl-CoA to form 3-ketoacyl-CoA or with malonyl-ACP to form 3-ketoacyl-ACP. This reaction is a key step in the fatty acid synthesis cycle, as the resulting acyl chain is two carbon atoms longer than before. KSs exist as individual enzymes, as they do in type II fatty acid synthesis and type II polyketide synthesis, or as domains in large multidomain enzymes, such as type I fatty acid synthases (FASs) and polyketide synthases (PKSs). KSs are divided into five families: KS1, KS2, KS3, KS4, and KS5.
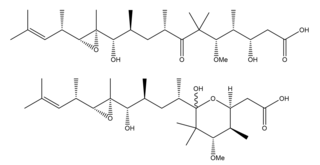
Gephyronic acid is a polyketide that exists as an equilibrating mixture of structural isomers. In nature, gephyronic acid is produced by slow growing myxobacterium: Archangium gephyra strain Ar3895 and Cystobacter violaceus strain Cb vi76. It is the first antibiotic in myxobacteria that was reported to specifically inhibit eukaryotic protein synthesis.

Swinholides are dimeric 42 carbon-ring polyketides that exhibit a 2-fold axis of symmetry. Found mostly in the marine sponge Theonella, swinholides encompass cytotoxic and antifungal activities via disruption of the actin skeleton. Swinholides were first described in 1985 and the structure and stereochemistry were updated in 1989 and 1990, respectively. Thirteen swinholides have been described in the literature, including close structural compounds such as misakinolides/bistheonellides, ankaraholides, and hurgholide A It is suspected that symbiotic microbes that inhabit the sponges rather than the sponges themselves produce swinholides since the highest concentration of swinholides are found in the unicellular bacterial fraction of sponges and not in the sponge fraction or cyanobacteria fraction that also inhabit the sponges.

Annimycin is a polyenoic acid amide natural product produced by Streptomyces calvus. Annimycin inhibits the sporulation of several actinobacterial genera.

Dihydromaltophilin, or heat stable anti-fungal factor (HSAF), is a secondary metabolite of Streptomyces sp. and Lysobacter enzymogenes. HSAF is a polycyclic tetramate lactam containing a single tetramic acid unit and a 5,5,6-tricyclic system. HSAF has been shown to have anti-fungal activity mediated through the disruption of the biosynthesis of Sphingolipid's by targeting a ceramide synthase unique to fungi.