
Invagination is the process of a surface folding in on itself to form a cavity, pouch or tube. In developmental biology, invagination of epithelial sheets occurs in many contexts during embryonic development. Invagination is critical for making the primitive gut during gastrulation in many organisms, forming the neural tube in vertebrates, and in the morphogenesis of countless organs and sensory structures. Models of invagination that have been most thoroughly studied include the ventral furrow in Drosophilamelanogaster, neural tube formation, and gastrulation in many marine organisms. The cellular mechanisms of invagination vary from one context to another but at their core they involve changing the mechanics of one side of a sheet of cells such that this pressure induces a bend in the tissue.
In cellular biology, the Wnt signaling pathways are a group of signal transduction pathways which begin with proteins that pass signals into a cell through cell surface receptors. The name Wnt, pronounced "wint", is a portmanteau created from the names Wingless and Int-1. Wnt signaling pathways use either nearby cell-cell communication (paracrine) or same-cell communication (autocrine). They are highly evolutionarily conserved in animals, which means they are similar across animal species from fruit flies to humans.

Cell junctions or junctional complexes are a class of cellular structures consisting of multiprotein complexes that provide contact or adhesion between neighboring cells or between a cell and the extracellular matrix in animals. They also maintain the paracellular barrier of epithelia and control paracellular transport. Cell junctions are especially abundant in epithelial tissues. Combined with cell adhesion molecules and extracellular matrix, cell junctions help hold animal cells together.

Angiomotin (AMOT) is a protein that in humans is encoded by the AMOT gene. It belongs to the motin family of angiostatin binding proteins, which includes angiomotin, angiomotin-like 1 (AMOTL1) and angiomotin-like 2 (AMOTL2) characterized by coiled-coil domains at N-terminus and consensus PDZ-binding domain at the C-terminus. Angiomotin is expressed predominantly in endothelial cells of capillaries as well as angiogenic tissues such as placenta and solid tumor.

Tight junctions, also known as occluding junctions or zonulae occludentes, are multiprotein junctional complexes between epithelial cells,, sealing and preventing leakage of solutes and water. They also play a critical role maintaining the structure and permeability of endothelial cells. Tight junctions may also serve as leaky pathways by forming selective channels for small cations, anions, or water. The corresponding junctions that occur in invertebrates are septate junctions.
Prickle is also known as REST/NRSF-interacting LIM domain protein, which is a putative nuclear translocation receptor. Prickle is part of the non-canonical Wnt signaling pathway that establishes planar cell polarity. A gain or loss of function of Prickle1 causes defects in the convergent extension movements of gastrulation. In epithelial cells, Prickle2 establishes and maintains cell apical/basal polarity. Prickle1 plays an important role in the development of the nervous system by regulating the movement of nerve cells.

α-Catenin (alpha-catenin) functions as the primary protein link between cadherins and the actin cytoskeleton. It has been reported that the actin binding proteins vinculin and α-actinin can bind to alpha-catenin. It has been suggested that alpha-catenin does not bind with high affinity to both actin filaments and the E-cadherin-beta-catenin complex at the same time. It has been observed that when α-catenin is not in a molecular complex with β-catenin, it dimerizes and functions to regulate actin filament assembly, possibly by competing with Arp2/3 protein. α-Catenin exhibits significant protein dynamics. However, a protein complex including a cadherin, actin, β-catenin and α-catenin has not been isolated.

Cell division control protein 42 homolog is a protein that in humans is encoded by the CDC42 gene. Cdc42 is involved in regulation of the cell cycle. It was originally identified in S. cerevisiae (yeast) as a mediator of cell division, and is now known to influence a variety of signaling events and cellular processes in a variety of organisms from yeast to mammals.

YAP1, also known as YAP or YAP65, is a protein that acts as a transcription coregulator that promotes transcription of genes involved in cellular proliferation and suppressing apoptotic genes. YAP1 is a component in the hippo signaling pathway which regulates organ size, regeneration, and tumorigenesis. YAP1 was first identified by virtue of its ability to associate with the SH3 domain of Yes and Src protein tyrosine kinases. YAP1 is a potent oncogene, which is amplified in various human cancers.

SCRIB, also known as Scribble, SCRIBL, or Scribbled homolog (Drosophila), is a scaffold protein which in humans is encoded by the SCRIB gene. It was originally isolated in Drosophila melanogaster in a pathway (also known as the Scribble complex) with DLGAP5 (Discs large) and LLGL1 (Lethal giant larvae) as a tumor suppressor. In humans, SCRIB is found as a membrane protein and is involved in cell migration, cell polarity, and cell proliferation in epithelial cells. There is also strong evidence that SCRIB may play a role in cancer progression because of its strong homology to the Drosophila protein.

Partitioning defective 6 homolog alpha is a protein that in humans is encoded by the PARD6A gene.

Planar cell polarity (PCP) is the protein-mediated signaling that coordinates the orientation of cells in a layer of epithelial tissue. In vertebrates, examples of mature PCP oriented tissue are the stereo-cilia bundles in the inner ear, motile cilia of the epithelium, and cell motility in epidermal wound healing. Additionally, PCP is known to be crucial to major developmental time points including coordinating convergent extension during gastrulation and coordinating cell behavior for neural tube closure. Cells orient themselves and their neighbors by establishing asymmetric expression of PCP components on opposing cell members within cells to establish and maintain the directionality of the cells. Some of these PCP components are transmembrane proteins which can proliferate the orientation signal to the surrounding cells.
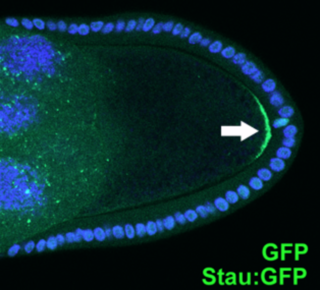
Cell polarity refers to spatial differences in shape, structure, and function within a cell. Almost all cell types exhibit some form of polarity, which enables them to carry out specialized functions. Classical examples of polarized cells are described below, including epithelial cells with apical-basal polarity, neurons in which signals propagate in one direction from dendrites to axons, and migrating cells. Furthermore, cell polarity is important during many types of asymmetric cell division to set up functional asymmetries between daughter cells.

The Hippo signaling pathway, also known as the Salvador-Warts-Hippo (SWH) pathway, is a signaling pathway that controls organ size in animals through the regulation of cell proliferation and apoptosis. The pathway takes its name from one of its key signaling components—the protein kinase Hippo (Hpo). Mutations in this gene lead to tissue overgrowth, or a "hippopotamus"-like phenotype.
Epithelial polarity is one example of the cell polarity that is a fundamental feature of many types of cells. Epithelial cells feature distinct 'apical', 'lateral' and 'basal' plasma membrane domains. Epithelial cells connect to one another via their lateral membranes to form epithelial sheets that line cavities and surfaces throughout the animal body. Each plasma membrane domain has a distinct protein composition, giving them distinct properties and allowing directional transport of molecules across the epithelial sheet. How epithelial cells generate and maintain polarity remains unclear, but certain molecules have been found to play a key role.
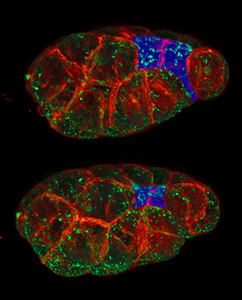
In morphogenesis, apical constriction is the process in which contraction of the apical side of a cell causes the cell to take on a wedged shape. Generally, this shape change is coordinated across many cells of an epithelial layer, generating forces that can bend or fold the cell sheet.

In molecular biology, bantam microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.

Septate junctions are intercellular junctions found in invertebrate epithelial cells, appearing as ladder-like structures under electron microscopy. They are thought to provide structural strength and a barrier to solute diffusion through the intercellular space. They are considered somewhat analogous to the (vertebrate) tight junctions; however, tight and septate junctions are different in many ways. Known insect homologues of tight junction components are components of conserved signalling pathways that localize to either adherens junctions, the subapical complex, or the marginal zone. Recent studies show that septate junctions are also identified in the myelinated nerve fibers of the vertebrates.

Madin-Darby canine kidney (MDCK) cells are a model mammalian cell line used in biomedical research. MDCK cells are used for a wide variety of cell biology studies including cell polarity, cell-cell adhesions, collective cell motility, toxicity studies, as well as responses to growth factors. It is one of few cell culture models that is suited for 3D cell culture and multicellular rearrangements known as branching morphogenesis.

Cell extrusion, discovered in 2001, is a process conserved in epithelial from humans to sea sponge to seamlessly remove unwanted or dying cells while maintaining the integrity of the epithelial barrier. If cells were to die without extrusion, gaps would be created, compromising the epithelia's function. While cell targeted to die by apoptotic stimuli extrude to prevent gaps from forming, most cells die as a result of extruding live cells. To maintain epithelial cell number homeostasis, live cells extrude when they become too crowded.
















