 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
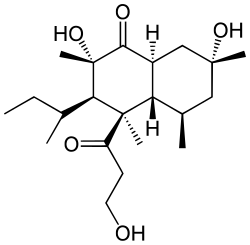
| Formula | C21H36O5 |
| Molar mass | 368.514 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 103.5 to 108 °C (218.3 to 226.4 °F) |
| |
| |
| (verify) | |
Betaenone B, like other betaenones (A and C), is a secondary metabolite isolated from the fungus Pleospora betae , a plant pathogen. [1] Its phytotoxic properties have been shown to cause sugar beet leaf spots, [1] [2] [3] which is characterized by black, pycnidia containing, concentric circles eventually leading to necrosis of the leaf tissue. [4] Of the seven phytotoxins isolated in fungal leaf spots from sugar beet (Beta vulgaris), betaenone B showed the least amount of phytotoxicity showing only 8% inhibition of growth while betaenone A and C showed 73% and 89% growth inhibition, respectively. [5] Betaenone B is therefore not considered toxic to the plant, but will produce leaf spots when present in high concentrations (0.33 μg/μL). [5] While the mechanism of action of betaenone B has yet to be elucidated, betaenone C has been shown to inhibit RNA and protein synthesis. [5] Most of the major work on betaenone B, including the initial structure elucidation of betaenone A, B and C as well as the partial elucidation mechanism of biosynthesis, was presented in three short papers published between 1983 and 1988. [1] [2] [3] The compounds were found to inhibit a variety of protein kinases signifying a possible role in cancer treatment. [6]
The structure of betaenone B was determined via nuclear magnetic resonance spectroscopy (NMR), CD[ clarification needed ] and optical rotatory dispersion (ORD) measurements. [1] While it was also shown that betaenone B could be converted to betaenone A by oxidation by PCC followed by exposure to base, [1] it wasn't until 1988 that a semi-complete total synthesis was reported. [7] Using 1,3-butadiene as a starting material, a stereoselective synthesis of (+/-)-4-de(3'-hydroxypropionyl) betanenone B was achieved in a 24-step synthesis. Bioactivity of this synthetic product was not tested and no further work on total synthesis of betaenones has been published since.
While a complete de novo synthesis of betaenone B has yet to be reported, Daniel Pratt and Paul Hopkins in 1988 proposed a method for synthesizing a precursor of betaenone B from methoxybenzoquinone and 1,3-butadiene via a Diels–Alder reaction and Claisen chemistry [ clarification needed ]. [7]

