In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐CH2‐COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a Clostridium tetani infection) can cause spastic paralysis due to uninhibited muscle contraction.
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). However, pI is also used. For brevity, this article uses pI. The net charge on the molecule is affected by pH of its surrounding environment and can become more positively or negatively charged due to the gain or loss, respectively, of protons (H+).
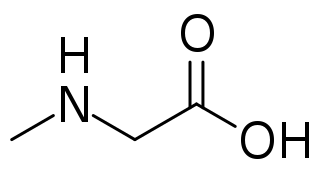
Sarcosine, also known as N-methylglycine, or monomethylglycine, is a amino acid with the formula CH3N(H)CH2CO2H. It exists at neutral pH as the zwitterion CH3N+(H)2CH2CO2−, which can be obtained as a white, water-soluble powder. Like some amino acids, sarcosine converts to a cation at low pH and an anion at high pH, with the respective formulas CH3N+(H)2CH2CO2H and CH3N(H)CH2CO2−. Sarcosine is a close relative of glycine, with a secondary amine in place of the primary amine.
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base. An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid.

β-Alanine is a naturally occurring beta amino acid, which is an amino acid in which the amino group is attached to the β-carbon instead of the more usual α-carbon for alanine (α-alanine). The IUPAC name for β-alanine is 3-aminopropanoic acid. Unlike its counterpart α-alanine, β-alanine has no stereocenter.
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit the wide range of possible labelling methods. Due to the strong affinity between biotin and streptavidin, the purification of biotinylated proteins has been a widely used approach to identify protein-protein interactions and post-translational events such as ubiquitylation in molecular biology.

Ethanolamine is an organic chemical compound with the formula HOCH
2CH
2NH
2 or C
2H
7NO. The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid with an odor reminiscent of ammonia. ETA molecules are a component in the formation of cellular membranes and are thus a molecular building block for life. It was thought to exist only on Earth and on certain asteroids, but in 2021 evidence was found that ETA molecules exist in interstellar space.

N-Methylethanolamine is an alkanolamine with the formula CH3NHCH2CH2OH. It is flammable, corrosive, colorless, viscous liquid. It is an intermediate in the biosynthesis of choline.
Methylamine is an organic compound with a formula of CH3NH2. This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine.

Piperazine is an organic compound that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring. Piperazine exists as small alkaline deliquescent crystals with a saline taste.
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base.
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.
Ethylamine, also known as ethanamine, is an organic compound with the formula CH3CH2NH2. This colourless gas has a strong ammonia-like odor. It condenses just below room temperature to a liquid miscible with virtually all solvents. It is a nucleophilic base, as is typical for amines. Ethylamine is widely used in chemical industry and organic synthesis.
Good's buffers are twenty buffering agents for biochemical and biological research selected and described by Norman Good and colleagues during 1966–1980. Most of the buffers were new zwitterionic compounds prepared and tested by Good and coworkers for the first time, though some were known compounds previously overlooked by biologists. Before Good's work, few hydrogen ion buffers between pH 6 and 8 had been accessible to biologists, and very inappropriate, toxic, reactive and inefficient buffers had often been used. Many Good's buffers became and remain crucial tools in modern biological laboratories.

Tricine is an organic compound that is used in buffer solutions. The name tricine comes from tris and glycine, from which it was derived. It is a white crystalline powder that is moderately soluble in water. It is a zwitterionic amino acid that has a pKa1 value of 2.3 at 25 °C, while its pKa2 at 20 °C is 8.15. Its useful buffering range of pH is 7.4-8.8. Along with bicine, it is one of Good's buffering agents. Good first prepared tricine to buffer chloroplast reactions.

Methyl diethanolamine, also known as N-methyl diethanolamine and more commonly as MDEA, is the organic compound with the formula CH3N(C2H4OH)2. It is a colorless liquid with an ammonia odor. It is miscible with water, ethanol and benzene. A tertiary amine, it is widely used as a sweetening agent in chemical, oil refinery, syngas production and natural gas.

Glycylglycine is the dipeptide of glycine, making it the simplest peptide. The compound was first synthesized by Emil Fischer and Ernest Fourneau in 1901 by boiling 2,5-diketopiperazine with hydrochloric acid. Shaking with alkali and other synthesis methods have been reported.
Aminoacetonitrile is the organic compound with the formula NCCH2NH2. The compound is a colorless liquid. It is unstable at room temperature, owing to the incompatibility of the amine nucleophile and the nitrile electrophile. For this reason it is usually encountered as the chloride and bisulfate salts of the ammonium derivative, i.e., [NCCH2NH3]+Cl− and [NCCH2NH3]+HSO4−.









