
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula C4H4 and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term tetrahedranes is used to describe a class of molecules and ions with related structure, e.g. white phosphorus.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure R−CH2−C6H5. Benzyl features a benzene ring attached to a methylene group group.
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement where the net result is one pi bond being converted into one sigma bond or vice versa. These reactions are usually categorized by the following criteria:
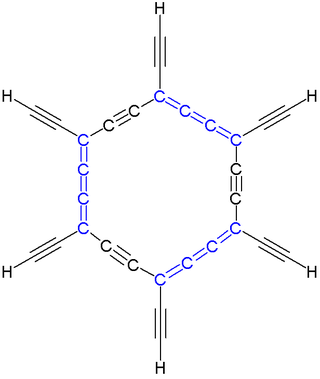
In organic chemistry, a carbo-mer (often carbo-mer or carbomer) is an expanded molecule obtained by insertion of C2 units into a given molecule. Carbo-mers differ from their templates in size but not in symmetry when each C–C single bond is replaced by an alkyne bond C-C≡C-C, each C=C double bond is replaced by an allene bond C=C=C=C, and each C≡C triple bond is replaced by C≡C-C≡C. The size of the carbo-mer continues to increase when more C2 units are inserted, so carbo-mers are also called carbon-molecules, where "n" is the number of acetylene or allene groups in an n-expansion unit. This concept, devised by Rémi Chauvin in 1995, is aimed at introducing new chemical properties for existing chemical motifs.
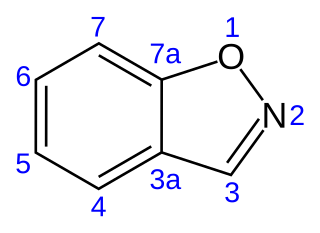
1,2-Benzisoxazole is an aromatic organic compound with a molecular formula C7H5NO containing a benzene-fused isoxazole ring structure. The compound itself has no common applications; however, functionalized benzisoxazoles and benzisoxazoyls have a variety of uses, including pharmaceutical drugs such as some antipsychotics (including risperidone, paliperidone, ocaperidone, and iloperidone) and the anticonvulsant zonisamide.
The Barton–Kellogg reaction is a coupling reaction between a diazo compound and a thioketone, giving an alkene by way of an episulfide intermediate. The Barton–Kellogg reaction is also known as Barton–Kellogg olefination and Barton olefin synthesis.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.

Schwartz's reagent is the common name for the organozirconium compound with the formula (C5H5)2ZrHCl, sometimes called zirconocene hydrochloride or zirconocene chloride hydride, and is named after Jeffrey Schwartz, a chemistry professor at Princeton University.This metallocene is used in organic synthesis for various transformations of alkenes and alkynes.
The Wurtz–Fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855, involving the formation of a new carbon-carbon bond by coupling two alkyl halides. Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. This modification of the Wurtz reaction is considered a separate process and is named for both scientists.
The Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product. The mechanism of the reaction was proposed in its original disclosure by A.G. Brook with further evidence later supplied by George M. Rubottom. After a Prilezhaev-type oxidation of the silyl enol ether with the peroxyacid to form the siloxy oxirane intermediate, acid-catalyzed ring-opening yields an oxocarbenium ion. This intermediate then participates in a 1,4-silyl migration to give an α-siloxy carbonyl derivative that can be readily converted to the α-hydroxy carbonyl compound in the presence of acid, base, or a fluoride source.

Dewar benzene (also spelled dewarbenzene) or bicyclo[2.2.0]hexa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures in 1869. However, he did not propose it as the structure of benzene, and in fact he supported the correct structure previously proposed by August Kekulé in 1865.
Spiropentadiene, or bowtiediene, is a hydrocarbon with formula C5H4. The simplest spiro-connected cycloalkene, it is very unstable—decomposing even below −100 °C—due to its high bond strain and does not occur in nature. Its synthesis was reported in 1991.
The Abramov reaction is the related conversions of trialkyl to α-hydroxy phosphonates by the addition to carbonyl compounds. In terms of mechanism, the reaction involves attack of the nucleophilic phosphorus atom on the carbonyl carbon. It was named after the Russian chemist Vasilii Semenovich Abramov (1904–1968) in 1957.
The imine Diels–Alder reaction involves the transformation of all-carbon dienes and imine dienophiles into tetrahydropyridines.

Perfluorobutanesulfonyl fluoride (nonafluorobutanesulfonyl fluoride, NfF) is a colorless, volatile liquid that is immiscible with water but soluble in common organic solvents. It is prepared by the electrochemical fluorination of sulfolane. NfF serves as an entry point to nonafluorobutanesulfonates (nonaflates), which are valuable as electrophiles in palladium catalyzed cross coupling reactions. As a perfluoroalkylsulfonylating agent, NfF offers the advantages of lower cost and greater stability over the more frequently used triflic anhydride. The fluoride leaving group is readily substituted by nucleophiles such as amines, phenoxides, and enolates, giving sulfonamides, aryl nonaflates, and alkenyl nonaflates, respectively. However, it is not attacked by water (in which it is stable at pH<12). Hydrolysis by barium hydroxide gives Ba(ONf)2, which upon treatment with sulfuric acid gives perfluorobutanesulfonic acid and insoluble barium sulfate.
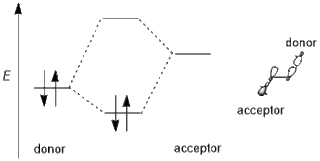
In chemistry, primarily organic and computational chemistry, a stereoelectronic effect is an effect on molecular geometry, reactivity, or physical properties due to spatial relationships in the molecules' electronic structure, in particular the interaction between atomic and/or molecular orbitals. Phrased differently, stereoelectronic effects can also be defined as the geometric constraints placed on the ground and/or transition states of molecules that arise from considerations of orbital overlap. Thus, a stereoelectronic effect explains a particular molecular property or reactivity by invoking stabilizing or destabilizing interactions that depend on the relative orientations of electrons in space.
David Markham Lemal is the Albert W. Smith Professor of Chemistry Emeritus and Research Professor of Chemistry at Dartmouth College. He received an A.B. degree (summa) from Amherst College in 1955 and a Ph.D. in Chemistry from Harvard University in 1959. At Harvard he worked with R. B. Woodward on deoxy sugars and a synthesis of the alkaloid yohimbine.

DMTMM is an organic triazine derivative commonly used for activation of carboxylic acids, particularly for amide synthesis. Amide coupling is one of the most common reactions in organic chemistry and DMTMM is one reagent used for that reaction. The mechanism of DMTMM coupling is similar to other common amide coupling reactions involving activated carboxylic acids. Its precursor, 2-chloro-4,6,-dimethoxy-1,3,5-triazine (CDMT), has also been used for amide coupling. DMTMM has also been used to synthesize other carboxylic functional groups such as esters and anhydrides. DMTMM is usually used in the chloride form but the tetrafluoroborate salt is also commercially available.

1,2,4,5-Tetrabromobenzene is an organobromine compound with the formula C6H2Br4. It is one of three isomers of tetrabromobenzene. The compound is a white solid. 1,2,4,5-Tetrabromobenzene is an important metabolite of the flame retardant hexabromobenzene.
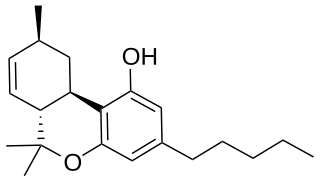
Delta-7-Tetrahydrocannabinol is a synthetic isomer of tetrahydrocannabinol. The (6aR,9S,10aR)-Δ7-THC epimer is only slightly less potent than Δ9-THC itself, while the (9R) enantiomer is much less potent.














