Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.

Geobacter is a genus of bacteria. Geobacter species are anaerobic respiration bacterial species which have capabilities that make them useful in bioremediation. Geobacter was found to be the first organism with the ability to oxidize organic compounds and metals, including iron, radioactive metals, and petroleum compounds into environmentally benign carbon dioxide while using iron oxide or other available metals as electron acceptors. Geobacter species are also found to be able to respire upon a graphite electrode. They have been found in anaerobic conditions in soils and aquatic sediment.
Microbial fuel cell (MFC) is a type of bioelectrochemical fuel cell system that generates electric current by diverting electrons produced from the microbial oxidation of reduced compounds on the anode to high-energy oxidized compounds such as oxygen on the cathode through an external electrical circuit. MFCs can be grouped into two general categories: mediated and unmediated. The first MFCs, demonstrated in the early 20th century, used a mediator: a chemical that transfers electrons from the bacteria in the cell to the anode. Unmediated MFCs emerged in the 1970s; in this type of MFC the bacteria typically have electrochemically active redox proteins such as cytochromes on their outer membrane that can transfer electrons directly to the anode. In the 21st century MFCs have started to find commercial use in wastewater treatment.

Shewanella is the sole genus included in the marine bacteria family Shewanellaceae. Some species within it were formerly classed as Alteromonas. Shewanella consists of facultatively anaerobic Gram-negative rods, most of which are found in extreme aquatic habitats where the temperature is very low and the pressure is very high. Shewanella bacteria are a normal component of the surface flora of fish and are implicated in fish spoilage. Shewanella chilikensis, a species of the genus Shewanella commonly found in the marine sponges of Saint Martin's Island of the Bay of Bengal, Bangladesh.
An enzymatic biofuel cell is a specific type of fuel cell that uses enzymes as a catalyst to oxidize its fuel, rather than precious metals. Enzymatic biofuel cells, while currently confined to research facilities, are widely prized for the promise they hold in terms of their relatively inexpensive components and fuels, as well as a potential power source for bionic implants.

Shewanella oneidensis is a bacterium notable for its ability to reduce metal ions and live in environments with or without oxygen. This proteobacterium was first isolated from Lake Oneida, NY in 1988, hence its name.
Many microorganisms can naturally grow together on surfaces to form complex aggregations called biofilms. Much research has been done on methods to remove biofilms in clinical and food manufacturing processes, but biofilms are also used for constructive purposes in a variety of industries. One distinctive characteristic of biofilm formation is that microorganisms within biofilms are often much tougher and more resistant to environmental stress compared to individual microorganisms. The cells are stationary and are able to adapt to adverse environments. This phenomenon of enhanced resistance can be beneficial in industrial chemical production where microorganisms within biofilms may tolerate higher chemical concentration and act as robust biorefineries for various products. These microbes have also been used in bioremediation to remove contaminants from freshwater and wastewater. More novel uses of biofilms include generating electricity using microbial fuel cells. Challenges to scaling up this technology include cost, controlling the growth of biofilms, and membrane fouling.

Bacterial nanowires are electrically conductive appendages produced by a number of bacteria most notably from the Geobacter and Shewanella genera. Conductive nanowires have also been confirmed in the oxygenic cyanobacterium Synechocystis PCC6803 and a thermophilic, methanogenic coculture consisting of Pelotomaculum thermopropionicum and Methanothermobacter thermoautotrophicus. From physiological and functional perspectives, bacterial nanowires are diverse. The precise role microbial nanowires play in their biological systems has not been fully realized, but several proposed functions exist. Outside of a naturally occurring environment, bacterial nanowires have shown potential to be useful in several fields, notably the bioenergy and bioremediation industries.
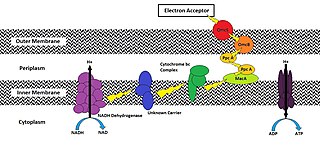
An exoelectrogen normally refers to a microorganism that has the ability to transfer electrons extracellularly. While exoelectrogen is the predominant name, other terms have been used: electrochemically active bacteria, anode respiring bacteria, and electricigens. Electrons exocytosed in this fashion are produced following ATP production using an electron transport chain (ETC) during oxidative phosphorylation. Conventional cellular respiration requires a final electron acceptor to receive these electrons. Cells that use molecular oxygen (O2) as their final electron acceptor are described as using aerobic respiration, while cells that use other soluble compounds as their final electron acceptor are described as using anaerobic respiration. However, the final electron acceptor of an exoelectrogen is found extracellularly and can be a strong oxidizing agent in aqueous solution or a solid conductor/electron acceptor. Two commonly observed acceptors are iron compounds (specifically Fe(III) oxides) and manganese compounds (specifically Mn(III/IV) oxides). As oxygen is a strong oxidizer, cells are able to do this strictly in the absence of oxygen.
A Bioelectrochemical reactor is a type of bioreactor where bioelectrochemical processes are used to degrade/produce organic materials using microorganisms. This bioreactor is divided in two parts: The anode, where the oxidation reaction takes place; And the cathode, where the reduction occurs. At these sites, electrons are passed to and from microbes to power reduction of protons, breakdown of organic waste, or other desired processes. They are used in microbial electrosynthesis, environmental remediation, and electrochemical energy conversion. Examples of bioelectrochemical reactors include microbial electrolysis cells, microbial fuel cells, enzymatic biofuel cells, electrolysis cells, microbial electrosynthesis cells, and biobatteries.
Microbial electrosynthesis (MES) is a form of microbial electrocatalysis in which electrons are supplied to living microorganisms via a cathode in an electrochemical cell by applying an electric current. The electrons are then used by the microorganisms to reduce carbon dioxide to yield industrially relevant products. The electric current would ideally be produced by a renewable source of power. This process is the opposite to that employed in a microbial fuel cell, in which microorganisms transfer electrons from the oxidation of compounds to an anode to generate an electric current.
Geobacter metallireducens is a gram-negative metal-reducing proteobacterium. It is a strict anaerobe that oxidizes several short-chain fatty acids, alcohols, and monoaromatic compounds with Fe(III) as the sole electron acceptor. It can also use uranium for its growth and convert U(VI) to U(IV).
Geobacter sulfurreducens is a gram-negative metal and sulphur-reducing proteobacterium. It is rod-shaped, obligately anaerobic, non-fermentative, has flagellum and type four pili, and is closely related to Geobacter metallireducens. Geobacter sulfurreducens is an anaerobic species of bacteria that comes from the family of bacteria called Geobacteraceae. Under the genus of Geobacter, G. sulfurreducens is one out of twenty different species. The Geobacter genus was discovered by Dr. Derek R. Lovley in 1987. G. sulfurreducens was first isolated in Norman, Oklahoma, USA from materials found around the surface of a contaminated ditch.
Geopsychrobacter electrodiphilus is a species of bacteria, the type species of its genus. It is a psychrotolerant member of its family, capable of attaching to the anodes of sediment fuel cells and harvesting electricity by oxidation of organic compounds to carbon dioxide and transferring the electrons to the anode.
Biological photovoltaics (BPV) is an energy-generating technology which uses oxygenic photoautotrophic organisms, or fractions thereof, to harvest light energy and produce electrical power. Biological photovoltaic devices are a type of biological electrochemical system, or microbial fuel cell, and are sometimes also called photo-microbial fuel cells or “living solar cells”. In a biological photovoltaic system, electrons generated by photolysis of water are transferred to an anode. A relatively high-potential reaction takes place at the cathode, and the resulting potential difference drives current through an external circuit to do useful work. It is hoped that using a living organism as the light harvesting material, will make biological photovoltaics a cost-effective alternative to synthetic light-energy-transduction technologies such as silicon-based photovoltaics.
OmcS oxidoreductase are enzymes found in some species of bacteria, including Geobacter sulfurreducens, where they catalyze the transfer of electrons. They are multiheme c-Type cytochromes localized outside of the cell along the pili of some exoelectrogenic bacterial species, serving as mediator of extracellular electron transfer from pili to Fe(III) oxides and other extracellular electron acceptors.
Dissimilatory metal-reducing microorganisms are a group of microorganisms (both bacteria and archaea) that can perform anaerobic respiration utilizing a metal as terminal electron acceptor rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration. The most common metals used for this end are iron [Fe(III)] and manganese [Mn(IV)], which are reduced to Fe(II) and Mn(II) respectively, and most microorganisms that reduce Fe(III) can reduce Mn(IV) as well. But other metals and metalloids are also used as terminal electron acceptors, such as vanadium [V(V)], chromium [Cr(VI)], molybdenum [Mo(VI)], cobalt [Co(III)], palladium [Pd(II)], gold [Au(III)], and mercury [Hg(II)].

Forest rings are large, circular patterns of low tree density in the boreal forests of northern Canada. These rings can range from 50 metres (160 ft) to nearly 2 kilometres (1.2 mi) in diameter, with rims about 20 metres (66 ft) in thickness. The origin of forest rings is not known, despite several mechanisms for their creation having been proposed. Such hypotheses include radially growing fungus, buried kimberlite pipes, trapped gas pockets, and meteorite impact craters.
A microbial desalination cell (MDC) is a biological electrochemical system that implements the use of electro-active bacteria to power desalination of water in situ, resourcing the natural anode and cathode gradient of the electro-active bacteria and thus creating an internal supercapacitor. Available water supply has become a worldwide endemic as only .3% of the earth's water supply is usable for human consumption, while over 99% is sequestered by oceans, glaciers, brackish waters, and biomass. Current applications in electrocoagulation, such as microbial desalination cells, are able to desalinate and sterilize formerly unavailable water to render it suitable for safe water supply. Microbial desalination cells stem from microbial fuel cells, deviating by no longer requiring the use of a mediator and instead relying on the charged components of the internal sludge to power the desalination process. Microbial desalination cells therefore do not require additional bacteria to mediate the catabolism of the substrate during biofilm oxidation on the anodic side of the capacitor. MDCs and other bio-electrical systems are favored over reverse osmosis, nanofiltration and other desalination systems due to lower costs, energy and environmental impacts associated with bio-electrical systems.

Gemma Reguera is a Spanish-American microbiologist and professor at Michigan State University. She is the editor-in-chief of the journal Applied and Environmental Microbiology and was elected fellow of the American Academy of Microbiology in 2019. She is the recipient of the 2022 Alice C. Evans Award for Advancement of Women from the American Society for Microbiology. Her lab's research is focused on electrical properties of metal-reducing microorganisms.







