
Phosphorus is a chemical element; it has symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram. In minerals, phosphorus generally occurs as phosphate.

In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, a.k.a. phosphoric acid H3PO4.

A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment or hand-tool methods.
In cooking, a leavening agent or raising agent, also called a leaven or leavener, is any one of a number of substances used in doughs and batters that cause a foaming action that lightens and softens the mixture. An alternative or supplement to leavening agents is mechanical action by which air is incorporated. Leavening agents can be biological or synthetic chemical compounds. The gas produced is often carbon dioxide, or occasionally hydrogen.
The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. Calcium phosphates are white solids of nutritional value and are found in many living organisms, e.g., bone mineral and tooth enamel. In milk, it exists in a colloidal form in micelles bound to casein protein with magnesium, zinc, and citrate–collectively referred to as colloidal calcium phosphate (CCP). Various calcium phosphate minerals are used in the production of phosphoric acid and fertilizers. Overuse of certain forms of calcium phosphate can lead to nutrient-containing surface runoff and subsequent adverse effects upon receiving waters such as algal blooms and eutrophication (over-enrichment with nutrients and minerals).

Phosphorite, phosphate rock or rock phosphate is a non-detrital sedimentary rock that contains high amounts of phosphate minerals. The phosphate content of phosphorite (or grade of phosphate rock) varies greatly, from 4% to 20% phosphorus pentoxide (P2O5). Marketed phosphate rock is enriched ("beneficiated") to at least 28%, often more than 30% P2O5. This occurs through washing, screening, de-liming, magnetic separation or flotation. By comparison, the average phosphorus content of sedimentary rocks is less than 0.2%.

Cupellation is a refining process in metallurgy in which ores or alloyed metals are treated under very high temperatures and subjected to controlled operations to separate noble metals, like gold and silver, from base metals, like lead, copper, zinc, arsenic, antimony, or bismuth, present in the ore. The process is based on the principle that precious metals typically oxidise or react chemically at much higher temperatures than base metals. When they are heated at high temperatures, the precious metals remain apart, and the others react, forming slags or other compounds.

Calcium phosphide (CP) is the inorganic compound with the formula Ca3P2. It is one of several phosphides of calcium, being described as the salt-like material composed of Ca2+ and P3−. Other, more exotic calcium phosphides have the formula CaP / Ca2P2, CaP3, and Ca5P8.

Tricalcium phosphate (sometimes abbreviated TCP), more commonly known as Calcium phosphate, is a calcium salt of phosphoric acid with the chemical formula Ca3(PO4)2. It is also known as tribasic calcium phosphate and bone phosphate of lime (BPL). It is a white solid of low solubility. Most commercial samples of "tricalcium phosphate" are in fact hydroxyapatite.

Monocalcium phosphate is an inorganic compound with the chemical formula Ca(H2PO4)2 ("AMCP" or "CMP-A" for anhydrous monocalcium phosphate). It is commonly found as the monohydrate ("MCP" or "MCP-M"), Ca(H2PO4)2·H2O. Both salts are colourless solids. They are used mainly as superphosphate fertilizers and are also popular leavening agents.
Superphosphate is a chemical fertiliser first synthesised in the 1840s by reacting bones with sulfuric acid. The process was subsequently improved by reacting phosphate coprolites with sulfuric acid. Subsequently, other phosphate-rich deposits such as phosphorite were discovered and used. Soluble phosphate is an essential nutrient for all plants, and the availability of superphosphate revolutionised agricultural productivity.
Aluminium phosphate is a chemical compound. In nature it occurs as the mineral berlinite. Many synthetic forms of aluminium phosphate are known. They have framework structures similar to zeolites and some are used as catalysts, ion-exchangers or molecular sieves. Commercial aluminium phosphate gel is available.
In metallurgy, refining consists of purifying an impure metal. It is to be distinguished from other processes such as smelting and calcining in that those two involve a chemical change to the raw material, whereas in refining, the final material is usually identical chemically to the original one, only it is purer. The processes used are of many types, including pyrometallurgical and hydrometallurgical techniques.
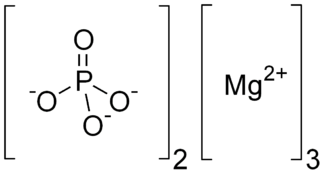
Trimagnesium phosphate describes inorganic compounds with formula Mg3(PO4)2.xH2O. They are magnesium acid salts of phosphoric acid, with varying amounts of water of crystallization: x = 0, 5, 8, 22.

Taranakite is a hydrated alkali iron-aluminium phosphate mineral with chemical formula (K,Na)3(Al,Fe3+)5(PO4)2(HPO4)6·18 H2O. It forms from the reaction of clay minerals or aluminous rocks with solutions enriched in phosphate derived from bat or bird guano or, less commonly, from bones or other organic matter. Taranakite is most commonly found in humid, bat inhabited caves near the boundary of guano layers with the cave surface. It is also found in perennially wet coastal locations that have been occupied by bird colonies. The type location, and its namesake, the Sugar Loaf Islands off Taranaki, New Zealand, is an example of a coastal occurrence.

Bioceramics and bioglasses are ceramic materials that are biocompatible. Bioceramics are an important subset of biomaterials. Bioceramics range in biocompatibility from the ceramic oxides, which are inert in the body, to the other extreme of resorbable materials, which are eventually replaced by the body after they have assisted repair. Bioceramics are used in many types of medical procedures. Bioceramics are typically used as rigid materials in surgical implants, though some bioceramics are flexible. The ceramic materials used are not the same as porcelain type ceramic materials. Rather, bioceramics are closely related to either the body's own materials or are extremely durable metal oxides.

A metallurgical assay is a compositional analysis of an ore, metal, or alloy, usually performed in order to test for purity or quality.

Copper(II) phosphate are inorganic compounds with the formula Cu3(PO4)2. They can be regarded as the cupric salts of phosphoric acid. Anhydrous copper(II) phosphate and a trihydrate are blue solids.
Zirconium phosphates (zirconium hydrogen phosphate) are acidic, inorganic cation exchange materials that have a layered structure with formula Zr(HPO4)2∙nH2O. These salts have high thermal and chemical stability, solid state ion conductivity, resistance to ionizing radiation, and the capacity to incorporate different types of molecules with different sizes between their layers. There are various phases of zirconium phosphate which vary in their interlaminar spaces and their crystalline structure. Among all the Zirconium phosphate phases the most widely used are the alpha (Zr(HPO4)2∙H2O) and the gamma (Zr(PO4)(H2PO4)∙2H2O) phase. The salts have been widely used in several applications such as: drug delivery, catalysis, nanocomposite, nuclear waste management, clinical dialyzer, among others.
Tetracalcium phosphate is the compound Ca4(PO4)2O, (4CaO·P2O5). It is the most basic of the calcium phosphates, and has a Ca/P ratio of 2, making it the most phosphorus poor phosphate. It is found as the mineral hilgenstockite, which is formed in industrial phosphate rich slag (called "Thomas slag"). This slag was used as a fertiliser due to the higher solubility of tetracalcium phosphate relative to apatite minerals. Tetracalcium phosphate is a component in some calcium phosphate cements that have medical applications.














