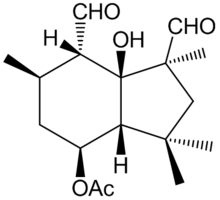
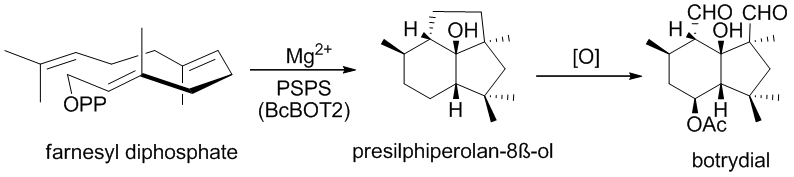
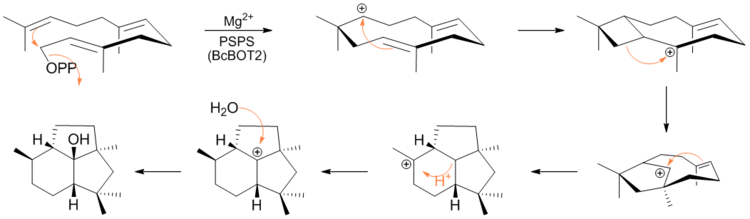
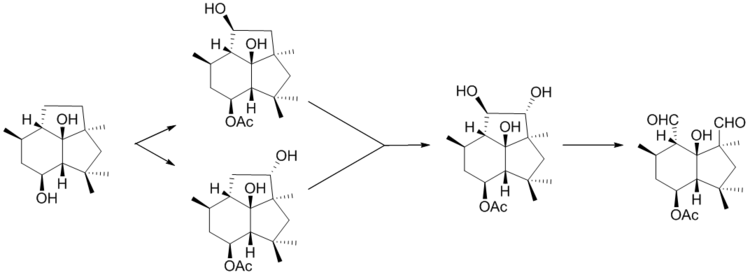
Botrytis cinerea is a necrotrophic fungus that affects many plant species, although its most notable hosts may be wine grapes. In viticulture, it is commonly known as "botrytis bunch rot"; in horticulture, it is usually called "grey mould" or "gray mold".

Abscisic acid (ABA) is a plant hormone. ABA functions in many plant developmental processes, including seed and bud dormancy, the control of organ size and stomatal closure. It is especially important for plants in the response to environmental stresses, including drought, soil salinity, cold tolerance, freezing tolerance, heat stress and heavy metal ion tolerance.

Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined, not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general phytoalexins are broad spectrum inhibitors; they are chemically diverse, and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids and alkaloids, however the term applies to any phytochemicals that are induced by microbial infection.

Caryophyllene, more formally (−)-β-caryophyllene, (BCP), is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa, rosemary, and hops. It is usually found as a mixture with isocaryophyllene and α-humulene, a ring-opened isomer. Caryophyllene is notable for having a cyclobutane ring, as well as a trans-double bond in a 9-membered ring, both rarities in nature.
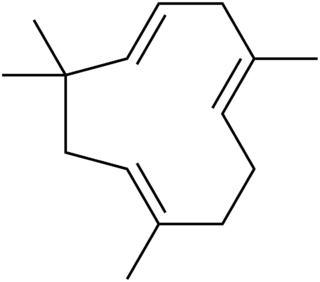
Humulene, also known as α-humulene or α-caryophyllene, is a naturally occurring monocyclic sesquiterpene (C15H24), containing an 11-membered ring and consisting of 3 isoprene units containing three nonconjugated C=C double bonds, two of them being triply substituted and one being doubly substituted. It was first found in the essential oils of Humulus lupulus (hops), from which it derives its name. Humulene is an isomer of β-caryophyllene, and the two are often found together as a mixture in many aromatic plants.

Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH. Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins.

The SKI protein is a nuclear proto-oncogene that is associated with tumors at high cellular concentrations. SKI has been shown to interfere with normal cellular functioning by both directly impeding expression of certain genes inside the nucleus of the cell as well as disrupting signaling proteins that activate genes.

Capsidiol is a terpenoid compound that accumulates in tobacco Nicotiana tabacum and chili pepper Capsicum annuum in response to fungal infection. Capsidiol is categorized under the broad term of phytoalexin, a class of low molecular weight plant secondary metabolites that are produced during infection. Phytoalexins are also characterized as a part of a two pronged response to infection which involves a short term response consisting of production of free radicals near the site of infection and a long term response involving the production of hormones and an increase in enzymes to biosynthesize phyoalexins such as capsidiol.

Juvabione, historically known as the paper factor, is the methyl ester of todomatuic acid, both of which are sesquiterpenes (C15) found in the wood of true firs of the genus Abies. They occur naturally as part of a mixture of sesquiterpenes based upon the bisabolane scaffold. Sesquiterpenes of this family are known as insect juvenile hormone analogues (IJHA) because of their ability to mimic juvenile activity in order to stifle insect reproduction and growth. These compounds play important roles in conifers as the second line of defense against insect induced trauma and fungal pathogens.

Capnellene is a naturally occurring tricyclic hydrocarbon derived from Capnella imbricata, a species of soft coral found in Indonesia. Since the 1970s, capnellene has been targeted for synthesis by numerous investigators due to its stereochemistry, functionality, and the interesting geometry of the carbon skeleton. Many alcohol derivatives of capnellene have demonstrated potential as a chemotherapeutic agent with antibacterial, anti-inflammatory and anti-tumor properties.

In molecular biology, this protein domain belongs to the terpene synthase family (TPS). Its role is to synthesize terpenes, which are part of primary metabolism, such as sterols and carotene, and also part of the secondary metabolism. This entry will focus on the N terminal domain of the TPS protein.

6-Methoxymellein is a dihydroisocoumarin, a phenolic compound found in carrots and carrot purées. It is responsible for bitterness in carrots. It is a phytoalexin, induced in carrot slices by UV-C, that allows resistance to Botrytis cinerea and other microorganisms.

3β-Androstanediol, also known as 5α-androstane-3β,17β-diol, and sometimes shortened in the literature to 3β-diol, is an endogenous steroid hormone and a metabolite of androgens like dehydroepiandrosterone (DHEA) and dihydrotestosterone (DHT).

Geosmin synthase or germacradienol-geosmin synthase designates a class of bifunctional enzymes that catalyze the conversion of farnesyl diphosphate (FPP) to geosmin, a volatile organic compound known for its earthy smell. The N-terminal half of the protein catalyzes the conversion of farnesyl diphosphate to germacradienol and germacrene D, followed by the C-terminal-mediated conversion of germacradienol to geosmin. The conversion of FPP to geosmin was previously thought to involve multiple enzymes in a biosynthetic pathway.

Cerevisterol (5α-ergosta-7,22-diene-3β,5,6β-triol) is a sterol. Originally described in the 1930s from the yeast Saccharomyces cerevisiae, it has since been found in several other fungi and, recently, in deep water coral. Cerevisterol has some in vitro bioactive properties, including cytotoxicity to some mammalian cell lines.
Presilphiperfolanol synthase (EC 4.2.3.74, BcBOT2, CND15) is an enzyme with systematic name (2E,6E)-farnesyl-diphosphate diphosphohydrolase (presilphiperfolan-8β-ol-forming). This enzyme catalyses the following chemical reaction

Thiophanate-methyl is an organic compound with the formula C6H4(NHC(S)NH(CO)OCH3)2. The compound is a colorless or white solid, although commercial samples are generally tan-colored. It is prepared from o-phenylenediamine.

5β-Dihydrotestosterone (5β-DHT), also known as 5β-androstan-17β-ol-3-one or as etiocholan-17β-ol-3-one, is an etiocholane (5β-androstane) steroid as well as an inactive metabolite of testosterone formed by 5β-reductase in the liver and bone marrow and an intermediate in the formation of 3α,5β-androstanediol and 3β,5β-androstanediol and, from them, respectively, etiocholanolone and epietiocholanolone. Unlike its isomer 5α-dihydrotestosterone, 5β-DHT either does not bind to or binds only very weakly to the androgen receptor. 5β-DHT is notable among metabolites of testosterone in that, due to the fusion of the A and B rings in the cis orientation, it has an extremely angular molecular shape, and this could be related to its lack of androgenic activity. 5β-DHT, unlike 5α-DHT, is also inactive in terms of neurosteroid activity, although its metabolite, etiocholanolone, does possess such activity.
Ulocladium botrytis is an anamorphic filamentous fungus belonging to the phylum Ascomycota. Commonly found in soil and damp indoor environments, U.botrytis is a hyphomycetous mould found in many regions of the world. It is also occasionally misidentified as a species of the genera Alternaria or Pithomyces due to morphological similarities. Ulocladium botrytis is rarely pathogenic to humans but is associated with human allergic responses and is used in allergy tests. Ulocladium botrytis has been implicated in some cases of human fungal nail infection. The fungus was first discovered in 1851 by German mycologist Carl Gottlieb Traugott Preuss.

Pyraclostrobin is a quinone outside inhibitor (QoI)-type fungicide used in agriculture. Among the QoIs, it lies within the strobilurin chemical class.