Related Research Articles

In cell biology, the centrosome is an organelle that serves as the main microtubule organizing center (MTOC) of the animal cell, as well as a regulator of cell-cycle progression. The centrosome provides structure for the cell. The centrosome is thought to have evolved only in the metazoan lineage of eukaryotic cells. Fungi and plants lack centrosomes and therefore use other structures to organize their microtubules. Although the centrosome has a key role in efficient mitosis in animal cells, it is not essential in certain fly and flatworm species.

A basal body is a protein structure found at the base of a eukaryotic undulipodium. The basal body was named by Theodor Wilhelm Engelmann in 1880. It is formed from a centriole and several additional protein structures, and is, essentially, a modified centriole. The basal body serves as a nucleation site for the growth of the axoneme microtubules. Centrioles, from which basal bodies are derived, act as anchoring sites for proteins that in turn anchor microtubules, and are known as the microtubule organizing center (MTOC). These microtubules provide structure and facilitate movement of vesicles and organelles within many eukaryotic cells.

Robert G. Roeder is an American biochemist. He is known as a pioneer scientist in eukaryotic transcription. He discovered three distinct nuclear RNA polymerases in 1969 and characterized many proteins involved in the regulation of transcription, including basic transcription factors and the first mammalian gene-specific activator over five decades of research. He is the recipient of the Gairdner Foundation International Award in 2000, the Albert Lasker Award for Basic Medical Research in 2003, and the Kyoto Prize in 2021. He currently serves as Arnold and Mabel Beckman Professor and Head of the Laboratory of Biochemical and Molecular Biology at The Rockefeller University.
Transcription factor II D (TFIID) is one of several general transcription factors that make up the RNA polymerase II preinitiation complex. RNA polymerase II holoenzyme is a form of eukaryotic RNA polymerase II that is recruited to the promoters of protein-coding genes in living cells. It consists of RNA polymerase II, a subset of general transcription factors, and regulatory proteins known as SRB proteins. Before the start of transcription, the transcription Factor II D (TFIID) complex binds to the core promoter DNA of the gene through specific recognition of promoter sequence motifs, including the TATA box, Initiator, Downstream Promoter, Motif Ten, or Downstream Regulatory elements.
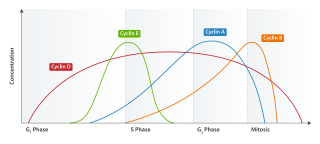
Cyclin E is a member of the cyclin family.
Nucleophosmin (NPM), also known as nucleolar phosphoprotein B23 or numatrin, is a protein that in humans is encoded by the NPM1 gene.

Serine/threonine-protein kinase PLK1, also known as polo-like kinase 1 (PLK-1) or serine/threonine-protein kinase 13 (STPK13), is an enzyme that in humans is encoded by the PLK1 gene.

Transcription factor E2F3 is a protein that in humans is encoded by the E2F3 gene.

Mitochondrial transcription factor A, abbreviated as TFAM or mtTFA, is a protein that in humans is encoded by the TFAM gene.

Activating transcription factor 5, also known as ATF5, is a protein that, in humans, is encoded by the ATF5 gene.

Afadin- and alpha-actinin-binding protein is a protein that in humans is encoded by the SSX2IP gene. It has been shown that it functions together with WDR8 in centrosome maturation, ensuring proper spindle length and orientation. The SSX2IP-WDR8 complex additionally promotes ciliary vesicle docking during ciliogenesis.

Centrosome-associated protein CEP250 is a protein that in humans is encoded by the CEP250 gene. This gene encodes a core centrosomal protein required for centriole-centriole cohesion during interphase of the cell cycle. The encoded protein dissociates from the centrosomes when parental centrioles separate at the beginning of mitosis. The protein associates with and is phosphorylated by NIMA-related kinase 2, which is also associated with the centrosome. Furthermore, CEP135 is also required for the centriolar localization of CEP250.

G2/mitotic-specific cyclin-F is a protein that in humans is encoded by the CCNF gene.

Centriolar coiled-coil protein of 110 kDa also known as centrosomal protein of 110 kDa or CP110 is a protein that in humans is encoded by the CCP110 gene. It is a cell cycle-dependent CDK substrate and regulates centrosome duplication. CP110 suppresses a cilia assembly program.
Robert Tjian is a Hong Kong-born American biochemist best known for his work on eukaryotic transcription. He is currently professor of biochemistry and molecular biology at the University of California, Berkeley and an Investigator of the Howard Hughes Medical Institute (HHMI). On April 1, 2009, Tjian became the President of HHMI. On August 4, 2015, he announced that he would step down as President at the end of 2016.
Mitochondrial biogenesis is the process by which cells increase mitochondrial numbers. It was first described by John Holloszy in the 1960s, when it was discovered that physical endurance training induced higher mitochondrial content levels, leading to greater glucose uptake by muscles. Mitochondrial biogenesis is activated by numerous different signals during times of cellular stress or in response to environmental stimuli, such as aerobic exercise.
Lewis C. Cantley is an American cell biologist and biochemist who has made significant advances to the understanding of cancer metabolism. Among his most notable contributions are the discovery and study of the enzyme PI-3-kinase, now known to be important to understanding cancer and diabetes mellitus. He is currently Meyer Director and Professor of Cancer Biology at the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine in New York City. He was formerly a professor in the Departments of Systems Biology and Medicine at Harvard Medical School, and the Director of Cancer Research at the Beth Israel Deaconess Medical Center, in Boston, Massachusetts. In 2016, he was elected Chairman of the Board for the Hope Funds for Cancer Research.

Centrosomal protein of 192 kDa, also known as Cep192, is a protein that in humans is encoded by the CEP192 gene. It is the homolog of the C. elegans and D. melanogaster gene SPD-2.

Spindle assembly abnormal protein 6 homolog (SAS-6) is a protein that in humans is encoded by the SASS6 gene.

Sharon Tooze, FMedSci is an American cell biologist who has made significant contributions to the field of autophagy. She is a senior scientist at the Francis Crick Institute and was awarded European Molecular Biology Organization (EMBO) membership in 2010.
References
- ↑ "Brian D. Dynlacht".
- ↑ Chen Z, Indjeian VB, McManus M, Wang L, Dynlacht BD (September 2002). "CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells". Dev. Cell. 3 (3): 339–50. doi: 10.1016/S1534-5807(02)00258-7 . PMID 12361598.
- ↑ Spektor A, Tsang WY, Khoo D, Dynlacht BD (August 2007). "CP110 suppresses a cilia assembly program". Cell. 130 (4): 678–90. doi: 10.1016/j.cell.2007.06.027 . PMID 17719545. S2CID 17974875.
- ↑ Li, J.; d'Angiolella, V.; Seeley, E. S.; Kim, S.; Kobayashi, T.; Fu, W.; Campos, E. I.; Pagano, M.; Dynlacht, B. D. (2013). "USP33 regulates centrosome biogenesis via deubiquitination of the centriolar protein CP110". Nature. 495 (7440): 255–259. Bibcode:2013Natur.495..255L. doi:10.1038/nature11941. PMC 3815529 . PMID 23486064.
- 1 2 Center for Oral History. "Brian D. Dynlacht". Science History Institute .
- 1 2 Van Benschoten, William (20 August 2004). Brian D. Dynlacht, Transcript of an Interview Conducted by William Van Benschoten at New York University New York City, New York on 19 and 20 August 2004 (PDF). Philadelphia, PA: Chemical Heritage Foundation.
- ↑ Dynlacht, Brian David; Hoey, Timothy; Tjian, Robert (Aug 1991). "Isolation of co-activators associated with the TATA-binding protein that mediate transcriptional activation". Cell. 66 (3): 563–576. doi:10.1016/0092-8674(81)90019-2. PMID 1907890. S2CID 5000858.
- ↑ Dynlacht BD, Flores O, Lees JA, Harlow E (August 1994). "Differential Regulation of E2F transactivation by cyclin/cdk2 complex". Genes & Development. 8 (15): 1172–86. doi: 10.1101/gad.8.15.1772 . PMID 7958856.