
Amyloids are aggregates of proteins characterised by a fibrillar morphology of typically 7–13 nm in diameter, a β-sheet secondary structure and ability to be stained by particular dyes, such as Congo red. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal structure and physiological functions (misfolding) and form fibrous deposits within and around cells. These protein misfolding and deposition processes disrupt the healthy function of tissues and organs.
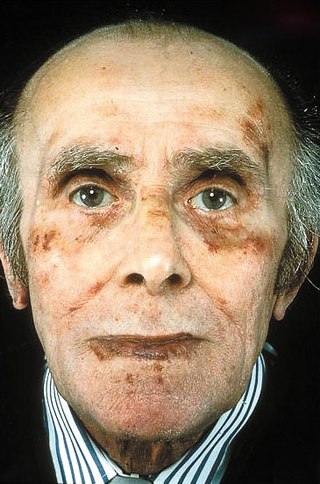
Amyloidosis is a group of diseases in which abnormal proteins, known as amyloid fibrils, build up in tissue. There are several non-specific and vague signs and symptoms associated with amyloidosis. These include fatigue, peripheral edema, weight loss, shortness of breath, palpitations, and feeling faint with standing. In AL amyloidosis, specific indicators can include enlargement of the tongue and periorbital purpura. In wild-type ATTR amyloidosis, non-cardiac symptoms include: bilateral carpal tunnel syndrome, lumbar spinal stenosis, biceps tendon rupture, small fiber neuropathy, and autonomic dysfunction.

Transthyretin (TTR or TBPA) is a transport protein in the plasma and cerebrospinal fluid that transports the thyroid hormone thyroxine (T4) and retinol to the liver. This is how transthyretin gained its name: transports thyroxine and retinol. The liver secretes TTR into the blood, and the choroid plexus secretes TTR into the cerebrospinal fluid.
Glycogen synthase kinase 3 (GSK-3) is a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues. First discovered in 1980 as a regulatory kinase for its namesake, glycogen synthase (GS), GSK-3 has since been identified as a protein kinase for over 100 different proteins in a variety of different pathways. In mammals, including humans, GSK-3 exists in two isozymes encoded by two homologous genes GSK-3α (GSK3A) and GSK-3β (GSK3B). GSK-3 has been the subject of much research since it has been implicated in a number of diseases, including type 2 diabetes, Alzheimer's disease, inflammation, cancer, addiction and bipolar disorder.
Amyloid beta denotes peptides of 36–43 amino acids that are the main component of the amyloid plaques found in the brains of people with Alzheimer's disease. The peptides derive from the amyloid-beta precursor protein (APP), which is cleaved by beta secretase and gamma secretase to yield Aβ in a cholesterol-dependent process and substrate presentation. Aβ molecules can aggregate to form flexible soluble oligomers which may exist in several forms. It is now believed that certain misfolded oligomers can induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a prion infection. The oligomers are toxic to nerve cells. The other protein implicated in Alzheimer's disease, tau protein, also forms such prion-like misfolded oligomers, and there is some evidence that misfolded Aβ can induce tau to misfold.

Molecular tweezers, and molecular clips, are host molecules with open cavities capable of binding guest molecules. The open cavity of the molecular tweezers may bind guests using non-covalent bonding which includes hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, and/or electrostatic effects. These complexes are a subset of macrocyclic molecular receptors and their structure is that the two "arms" that bind the guest molecule between them are only connected at one end leading to a certain flexibility of these receptor molecules.
Pittsburgh compound B (PiB) is a radioactive analog of thioflavin T, which can be used in positron emission tomography scans to image beta-amyloid plaques in neuronal tissue. Due to this property, Pittsburgh compound B may be used in investigational studies of Alzheimer's disease.

β2 microglobulin (B2M) is a component of MHC class I molecules. MHC class I molecules have α1, α2, and α3 proteins which are present on all nucleated cells. In humans, the β2 microglobulin protein is encoded by the B2M gene.

Thioflavins are fluorescent dyes that are available as at least two compounds, namely Thioflavin T and Thioflavin S. Both are used for histology staining and biophysical studies of protein aggregation. In particular, these dyes have been used since 1989 to investigate amyloid formation. They are also used in biophysical studies of the electrophysiology of bacteria. Thioflavins are corrosive, irritants, and are acutely toxic, causing serious eye damage. Thioflavin T has been used in research into Alzheimer's disease and other neurodegenerative diseases.

The serum amyloid P component (SAP) is the identical serum form of the amyloid P component (AP), a 25 kDa pentameric protein first identified as the pentagonal constituent of in vivo pathological deposits called "amyloid". APCS is its human gene.

Beta-secretase 1, also known as beta-site amyloid precursor protein cleaving enzyme 1, beta-site APP cleaving enzyme 1 (BACE1), membrane-associated aspartic protease 2, memapsin-2, aspartyl protease 2, and ASP2, is an enzyme that in humans is encoded by the BACE1 gene. Expression of BACE1 is observed mainly in neurons.

Pentraxins (PTX), also known as pentaxins, are an evolutionary conserved family of proteins characterised by containing a pentraxin protein domain. Proteins of the pentraxin family are involved in acute immunological responses. They are a class of pattern recognition receptors (PRRs). They are a superfamily of multifunctional conserved proteins, some of which are components of the humoral arm of innate immunity and behave as functional ancestors of antibodies (Abs). They are known as classical acute phase proteins (APP), known for over a century.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAbs) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. Antibodies are used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.

Cardiac amyloidosis is a subcategory of amyloidosis where there is depositing of the protein amyloid in the cardiac muscle and surrounding tissues. Amyloid, a misfolded and insoluble protein, can become a deposit in the heart's atria, valves, or ventricles. These deposits can cause thickening of different sections of the heart, leading to decreased cardiac function. The overall decrease in cardiac function leads to a plethora of symptoms. This multisystem disease was often misdiagnosed, with diagnosis previously occurring after death during autopsy. However, recent advancements of technologies have increased the diagnosis of the disease. Cardiac amyloidosis has multiple sub-types including light chain, familial, and senile. One of the most studied types is light chain cardiac amyloidosis. Prognosis depends on the extent of the deposits in the body and the type of amyloidosis. New treatment methods are actively being researched in regards to the treatment of heart failure and specific cardiac amyloidosis problems.

In medicine, proteinopathy, or proteopathy, protein conformational disorder, or protein misfolding disease, is a class of diseases in which certain proteins become structurally abnormal, and thereby disrupt the function of cells, tissues and organs of the body. Often the proteins fail to fold into their normal configuration; in this misfolded state, the proteins can become toxic in some way or they can lose their normal function. The proteinopathies include such diseases as Creutzfeldt–Jakob disease and other prion diseases, Alzheimer's disease, Parkinson's disease, amyloidosis, multiple system atrophy, and a wide range of other disorders. The term proteopathy was first proposed in 2000 by Lary Walker and Harry LeVine.

ETS Like-1 protein Elk-1 is a protein that in humans is encoded by the ELK1. Elk-1 functions as a transcription activator. It is classified as a ternary complex factor (TCF), a subclass of the ETS family, which is characterized by a common protein domain that regulates DNA binding to target sequences. Elk1 plays important roles in various contexts, including long-term memory formation, drug addiction, Alzheimer's disease, Down syndrome, breast cancer, and depression.

Serum amyloid A1 (SAA1) is a protein that in humans is encoded by the SAA1 gene. SAA1 is a major acute-phase protein mainly produced by hepatocytes in response to infection, tissue injury and malignancy. When released into blood circulation, SAA1 is present as an apolipoprotein associated with high-density lipoprotein (HDL). SAA1 is a major precursor of amyloid A (AA), the deposit of which leads to inflammatory amyloidosis.

Leukocyte cell-derived chemotaxin-2 (LECT2) is a protein first described in 1996 as a chemotactic factor for neutrophils, i.e. it stimulated human neutrophils to move directionally in an in vitro assay system. The protein was detected in and purified from cultures of Phytohaemagglutinin-activated human T-cell leukemia SKW-3 cells. Subsequent studies have defined LECT2 as a hepatokine, i.e. a substance made and released into the circulation by liver hepatocyte cells that regulates the function of other cells: it is a hepatocyte-derived, hormone-like, signaling protein.
AA amyloidosis is a form of amyloidosis, a disease characterized by the abnormal deposition of fibers of insoluble protein in the extracellular space of various tissues and organs. In AA amyloidosis, the deposited protein is serum amyloid A protein (SAA), an acute-phase protein which is normally soluble and whose plasma concentration is highest during inflammation.
Sir Mark Brian Pepys is a South African-born British academic of medicine. He was until 2011 Professor of Medicine at University College London and Head of Medicine at the Hampstead Campus and the Royal Free Hospital.

















