
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation with their smell.
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers.

Kerogen is solid, insoluble organic matter in sedimentary rocks. It consists of a variety of organic materials, including dead plants, algae, and other microorganisms, that have been compressed and heated by geological processes. Altogether kerogen is estimated to contain 1016 tons of carbon. This makes it the most abundant source of organic compounds on earth, exceeding the total organic content of living matter 10,000-fold.
Vitrinite is one of the primary components of coals and most sedimentary kerogens. Vitrinite is a type of maceral, where "macerals" are organic components of coal analogous to the "minerals" of rocks. Vitrinite has a shiny appearance resembling glass (vitreous). It is derived from the cell-wall material or woody tissue of the plants from which coal was formed. Chemically, it is composed of polymers, cellulose and lignin.
The abiogenic petroleum origin hypothesis proposes that most of earth's petroleum and natural gas deposits were formed inorganically. Scientific evidence overwhelmingly supports a biogenic origin for most of the worlds petroleum deposits. Mainstream theories about the formation of hydrocarbons on earth point to an origin from the decomposition of long-dead organisms, though the existence of hydrocarbons on extraterrestrial bodies like Saturn's moon Titan indicates that hydrocarbons are sometimes naturally produced by inorganic means. A historical overview of theories of the abiogenic origins of hydrocarbons has been published.

A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the incomplete combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.
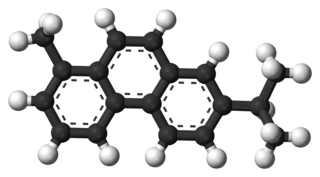
Retene, methyl isopropyl phenanthrene or 1-methyl-7-isopropyl phenanthrene, C18H18, is a polycyclic aromatic hydrocarbon present in the coal tar fraction, boiling above 360 °C. It occurs naturally in the tars obtained by the distillation of resinous woods. It crystallizes in large plates, which melt at 98.5 °C and boil at 390 °C. It is readily soluble in warm ether and in hot glacial acetic acid. Sodium and boiling amyl alcohol reduce it to a tetrahydroretene, but if it heated with phosphorus and hydriodic acid to 260 °C, a dodecahydride is formed. Chromic acid oxidizes it to retene quinone, phthalic acid and acetic acid. It forms a picrate that melts at 123-124 °C.
Epicuticular wax is a waxy coating which covers the outer surface of the plant cuticle in land plants. It may form a whitish film or bloom on leaves, fruits and other plant organs. Chemically, it consists of hydrophobic organic compounds, mainly straight-chain aliphatic hydrocarbons with or without a variety of substituted functional groups. The main functions of the epicuticular wax are to decrease surface wetting and moisture loss. Other functions include reflection of ultraviolet light, assisting in the formation of an ultra-hydrophobic and self-cleaning surface and acting as an anti-climb surface.
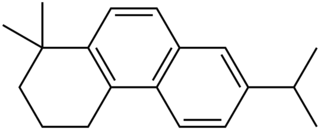
Simonellite (1,1-dimethyl-1,2,3,4-tetrahydro-7-isopropyl phenanthrene) is a polycyclic aromatic hydrocarbon with a chemical formula C19H24. It is similar to retene.

A carbon-to-nitrogen ratio is a ratio of the mass of carbon to the mass of nitrogen in organic residues. It can, amongst other things, be used in analysing sediments and soil including soil organic matter and soil amendments such as compost.
Phytane is the isoprenoid alkane formed when phytol, a constituent of chlorophyll, loses its hydroxyl group. When phytol loses one carbon atom, it yields pristane. Other sources of phytane and pristane have also been proposed than phytol.

Ferruginol is a natural phenol with a terpenoid substructure. Specifically, it is a diterpene of the abietane chemical class, meaning it is characterized by three fused six-membered rings and alkyl functional groups. Ferruginol was first identified in 1939 by Brandt and Neubauer as the main component in the resin of the Miro tree and has since been isolated from other conifer species in the families Cupressaceae and Podocarpaceae. As a biomarker, the presence of ferruginol in fossils, mainly resin, is used to describe the density of these conifers in that particular biosphere throughout time.
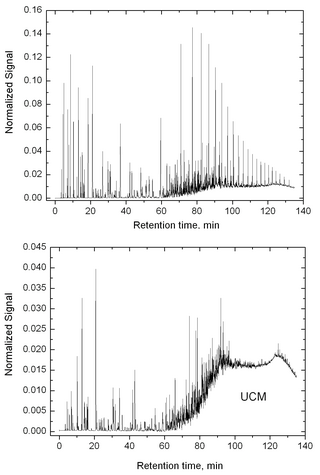
Unresolved complex mixture (UCM), or hump, is a feature frequently observed in gas chromatographic (GC) data of crude oils and extracts from organisms exposed to oil.
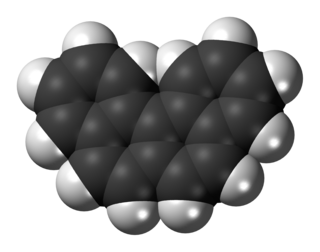
Benzo[c]phenanthrene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12. It is a white solid that is soluble in nonpolar organic solvents. It is a nonplanar molecule consisting of the fusion of four fused benzene rings. The compound is of mainly theoretical interest but it is environmentally occurring and weakly carcinogenic.
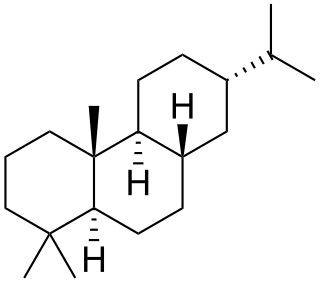
Abietane is a diterpene that forms the structural basis for a variety of natural chemical compounds such as abietic acid, carnosic acid, and ferruginol which are collectively known as abietanes or abietane diterpenes.

Dinosterol (4α,23,24-trimethyl-5α-cholest-22E-en-3β-ol) is a 4α-methyl sterol that is produced by several genera of dinoflagellates and is rarely found in other classes of protists. The steroidal alkane, dinosterane, is the 'molecular fossil' of dinosterol, meaning that dinosterane has the same carbon skeleton as dinosterol, but lacks dinosterol's hydroxyl group and olefin functionality. As such, dinosterane is often used as a biomarker to identify the presence of dinoflagelletes in sediments.
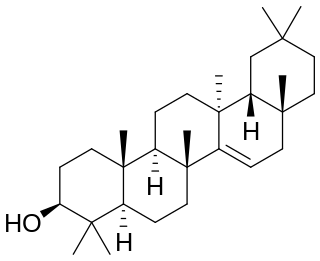
Taraxerol is a naturally-occurring pentacyclic triterpenoid. It exists in various higher plants, including Taraxacum officinale (Asteraceae), Alnus glutinosa (Betulaceae), Litsea dealbata (Lauraceae), Skimmia spp. (Rutaceae), Dorstenia spp. (Moraceae), Maytenus spp. (Celastraceae), and Alchornea latifolia (Euphobiaceae). Taraxerol was named "alnulin" when it was first isolated in 1923 from the bark of the grey alder by Zellner and Röglsperger. It also had the name "skimmiol" when Takeda and Yosiki isolated it from Skimmia (Rutaceae). A large number of medicinal plants are known to have this compound in their leaves, roots or seed oil.

Isoarborinol is a triterpenoid ubiquitously produced by angiosperms and is thus considered a biomarker for higher plants. Though no isoarborinol-producing microbe has been identified, isoarborinol is also considered a possible biomarker for marine bacteria, as its diagenetic end product, arborane, has been found in ancient marine sediments that predate the rise of plants. Importantly, isoarborinol may represent the phylogenetic link between hopanols and sterols.

Sugiol is a phenolic abietane derivative of ferruginol and can be used as a biomarker for specific families of conifers. The presence of sugiol can be used to identify the Cupressaceae s.1., podocarpaceae, and Araucaraiaceae families of conifers. The polar terpenoids are among the most resistant molecules to degradation besides n-alkanes and fatty acids, affording them high viability as biomarkers due to their longevity in the sedimentary record. Significant amounts of sugiol has been detected in fossil wood dated to the Eocene and Miocene periods, as well as a sample of Protopodocarpoxylon dated to the middle Jurassic.
Arborane is a class of pentacyclic triterpene consisting of organic compounds with four 6-membered rings and one 5-membered ring. Arboranes are thought to be derived from arborinols, a class of natural cyclic triterpenoids typically produced by flowering plants. Thus arboranes are used as a biomarker for angiosperms and cordaites. Arborane is a stereoisomer of a compound called fernane, the diagenetic product of fernene and fernenol. Because aborinol and fernenol have different biological sources, the ratio of arborane/fernane in a sample can be used to reconstruct a record for the relative abundances of different plants.















