Ascorbic acid is an organic compound with formula C
6H
8O
6, originally called hexuronic acid. It is a white solid, but impure samples can appear yellowish. It dissolves freely in water to give mildly acidic solutions. It is a mild reducing agent.

Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives, such as vinegar (pickling), salt (salting), smoke (smoking) and sugar (crystallization), have been used for centuries to preserve food. This allows for longer-lasting foods, such as bacon, sweets or wines.
The International Numbering System for Food Additives (INS) is an international naming system for food additives, aimed at providing a short designation of what may be a lengthy actual name. It is defined by Codex Alimentarius, the international food standards organisation of the World Health Organization (WHO) and Food and Agriculture Organization (FAO) of the United Nations (UN). The information is published in the document Class Names and the International Numbering System for Food Additives, first published in 1989, with revisions in 2008 and 2011. The INS is an open list, "subject to the inclusion of additional additives or removal of existing ones on an ongoing basis".

Stevia is a sweet sugar substitute that is about 50 to 300 times sweeter than sugar. It is extracted from the leaves of Stevia rebaudiana, a plant native to areas of Paraguay and Brazil. The active compounds in stevia are steviol glycosides. Stevia is heat-stable, pH-stable, and not fermentable. Humans cannot metabolize the glycosides in stevia, and it therefore has zero calories. Its taste has a slower onset and longer duration than that of sugar, and at high concentrations some of its extracts may have an aftertaste described as licorice-like or bitter. Stevia is used in sugar- and calorie-reduced food and beverage products as an alternative for variants with sugar.

E numbers, short for Europe numbers, are codes for substances used as food additives, including those found naturally in many foods, such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly found on food labels, their safety assessment and approval are the responsibility of the European Food Safety Authority (EFSA). The fact that an additive has an E number implies that its use was at one time permitted in products for sale in the European Single Market; some of these additives are no longer allowed today.
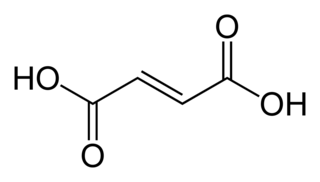
Fumaric acid or trans-butenedioic acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297. The salts and esters are known as fumarates. Fumarate can also refer to the C
4H
2O2−
4 ion (in solution). Fumaric acid is the trans isomer of butenedioic acid, while maleic acid is the cis isomer.

Canthaxanthin is a keto-carotenoid pigment widely distributed in nature. Carotenoids belong to a larger class of phytochemicals known as terpenoids. The chemical formula of canthaxanthin is C40H52O2. It was first isolated in edible mushrooms. It has also been found in green algae, bacteria, crustaceans, and bioaccumulates in fish such as carp, golden grey mullet, seabream and trush wrasse.

Sodium propanoate or sodium propionate is the sodium salt of propionic acid which has the chemical formula Na(C2H5COO). This white crystalline solid is deliquescent in moist air.

Apocarotenal, or trans-β-apo-8'-carotenal, is a carotenoid found in spinach and citrus fruits. Like other carotenoids, apocarotenal plays a role as a precursor of vitamin A, even though it has 50% less pro-vitamin A activity than β-carotene. The empirical chemical formula for apocarotenal is C30H40O.

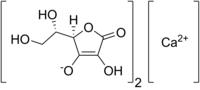
Mineral ascorbates are a group of salts of ascorbic acid. They are composed of a mineral cation bonded to ascorbate.

Ascorbyl palmitate is an ester formed from ascorbic acid and palmitic acid creating a fat-soluble form of vitamin C. In addition to its use as a source of vitamin C, it is also used as an antioxidant food additive. It is approved for use as a food additive in the EU, the U.S., Canada, Australia, and New Zealand.
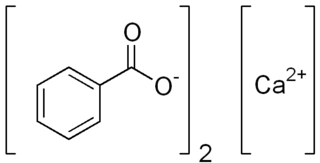
Calcium benzoate refers to the calcium salt of benzoic acid. When used in the food industry as a preservative, its E number is E213 ; it is approved for use as a food additive in the EU, USA and Australia and New Zealand.

Dimethyl dicarbonate (DMDC) is a colorless liquid with a pungent odor at high concentration at room temperature. It is primarily used as a beverage preservative, processing aid, or sterilant being highly active against typical beverage spoiling microorganisms like yeast, bacteria, or mould.

Sodium ascorbate is one of a number of mineral salts of ascorbic acid (vitamin C). The molecular formula of this chemical compound is C6H7NaO6. As the sodium salt of ascorbic acid, it is known as a mineral ascorbate. It has not been demonstrated to be more bioavailable than any other form of vitamin C supplement.

Potassium ascorbate is a compound with formula KC6H7O6. It is the potassium salt of ascorbic acid (vitamin C) and a mineral ascorbate. As a food additive, it has E number E303, INS number 303. Although it is not a permitted food additive in the UK, USA and the EU, it is approved for use in Australia and New Zealand. According to some studies, it has shown a strong antioxidant activity and antitumoral properties.

Potassium acetate (also called potassium ethanoate), (CH3COOK) is the potassium salt of acetic acid. It is a hygroscopic solid at room temperature.

Flavoxanthin is a natural xanthophyll pigment with a golden-yellow color found in small quantities in a variety of plants. As a food additive it used under the E number E161a as a food coloring although it is not approved for use in the EU or USA. It is listed as food additive 161a in Australia and New Zealand where it is approved for usage as an ingredient in food products.
Rubixanthin, or natural yellow 27, is a natural xanthophyll pigment with a red-orange color found in rose hips. As a food additive it used under the E number E161d as a food coloring; it is not approved for use in the USA or EU but is approved in Australia and New Zealand where it is listed as 161d.
Rhodoxanthin is a xanthophyll pigment with a purple color that is found in small quantities in a variety of plants including Taxus baccata and Lonicera morrowii. It is also found in the feathers of some birds. As a food additive it is used under the E number E161f as a food coloring. It is not approved for use in the EU or US; however, it is approved in Australia and New Zealand.
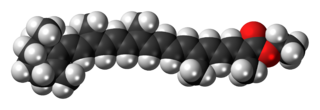
Food orange 7, the ethyl ester of beta-apo-8'-carotenic acid, is a carotenoid with an orange-red color. It is found in small quantities in some plants, but is often produced commercially from apocarotenal (E160e). It is used as a food coloring under the E number E160f and is approved for use in Australia and New Zealand. It was approved for use in the EU, but was delisted in November 2011 as it was no longer being manufactured.