Mineral ascorbates are a group of salts of ascorbic acid (vitamin C). [1] They are composed of a mineral cation bonded to ascorbate (the anion of ascorbic acid).

Mineral ascorbates are a group of salts of ascorbic acid (vitamin C). [1] They are composed of a mineral cation bonded to ascorbate (the anion of ascorbic acid).
Mineral ascorbates are powders manufactured by reacting ascorbic acid with mineral carbonates in aqueous solutions, venting the carbon dioxide, drying the reaction product, and then milling the dried product to the desired particle size.
The choice of the mineral carbonates can be calcium carbonate, potassium carbonate, sodium bicarbonate, magnesium carbonate, or many other mineral forms.
Mineral ascorbates are used as dietary supplements and food additives, and drugs. An example of a mineral ascorbate drug is sodium ascorbate injections (the acid form, ascorbic acid, of vitamin c is too acidic for injections).
Ascorbate salts may be better tolerated by the human body than the corresponding weakly acidic ascorbic acid.
Ascorbates are highly reactive antioxidants used as food preservatives. [2]
Examples of mineral ascorbates are:

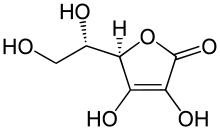
Ascorbic acid is an organic compound with formula C
6H
8O
6, originally called hexuronic acid. It is a white solid, but impure samples can appear yellowish. It dissolves freely in water to give mildly acidic solutions. It is a mild reducing agent.

Ash is the solid remnants of fires. Specifically, ash refers to all non-aqueous, non-gaseous residues that remain after something burns. In analytical chemistry, to analyse the mineral and metal content of chemical samples, ash is the non-gaseous, non-liquid residue after complete combustion.
A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or by undesirable chemical changes. In general, preservation is implemented in two modes, chemical and physical. Chemical preservation entails adding chemical compounds to the product. Physical preservation entails processes such as refrigeration or drying. Preservative food additives reduce the risk of foodborne infections, decrease microbial spoilage, and preserve fresh attributes and nutritional quality. Some physical techniques for food preservation include dehydration, UV-C radiation, freeze-drying, and refrigeration. Chemical preservation and physical preservation techniques are sometimes combined.

Vitamin C is a water-soluble vitamin found in citrus and other fruits, berries and vegetables. It is also a generic prescription medication and in some countries is sold as a non-prescription dietary supplement. As a therapy, it is used to prevent and treat scurvy, a disease caused by vitamin C deficiency.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the chloralkali process.

Magnesium carbonate, MgCO3, is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.

Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.
ATC code A12Mineral supplements is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup A12 is part of the anatomical group A Alimentary tract and metabolism.

Sodium benzoate also known as benzoate of soda is the sodium salt of benzoic acid, widely used as a food preservative (with an E number of E211) and a pickling agent. It appears as a white crystalline chemical with the formula C6H5COONa.
An anticaking agent is an additive placed in powdered or granulated materials, such as table salt or confectioneries, to prevent the formation of lumps (caking) and for easing packaging, transport, flowability, and consumption. Caking mechanisms depend on the nature of the material. Crystalline solids often cake by formation of liquid bridge and subsequent fusion of microcrystals. Amorphous materials can cake by glass transitions and changes in viscosity. Polymorphic phase transitions can also induce caking.

Ascorbyl palmitate is an ester formed from ascorbic acid and palmitic acid creating a fat-soluble form of vitamin C. In addition to its use as a source of vitamin C, it is also used as an antioxidant food additive. It is approved for use as a food additive in the EU, the U.S., Canada, Australia, and New Zealand.
Ascorbyl stearate (C24H42O7) is an ester formed from ascorbic acid and stearic acid. In addition to its use as a source of vitamin C, it is used as an antioxidant food additive in margarine (E number E305). The USDA limits its use to 0.02% individually or in conjunction with other antioxidants.

Sodium ascorbate is one of a number of mineral salts of ascorbic acid (vitamin C). The molecular formula of this chemical compound is C6H7NaO6. As the sodium salt of ascorbic acid, it is known as a mineral ascorbate. It has not been demonstrated to be more bioavailable than any other form of vitamin C supplement.

Calcium gluconate is the calcium salt of gluconic acid and is used as a mineral supplement and medication. As a medication it is used by injection into a vein to treat low blood calcium, high blood potassium, and magnesium toxicity. Supplementation is generally only required when there is not enough calcium in the diet. Supplementation may be done to treat or prevent osteoporosis or rickets. It can also be taken by mouth but is not recommended for injection into a muscle.
Magnesium aspartate is a magnesium salt of aspartic acid. It is used as a mineral supplement, and as an ingredient in manufacturing of cosmetics and household products.

A dough conditioner, flour treatment agent, improving agent or bread improver is any ingredient or chemical added to bread dough to strengthen its texture or otherwise improve it in some way. Dough conditioners may include enzymes, yeast nutrients, mineral salts, oxidants and reductants, bleaching agents and emulsifiers. They are food additives combined with flour to improve baking functionality. Flour treatment agents are used to increase the speed of dough rising and to improve the strength and workability of the dough.
Electrovite is manufactured by Dominion Veterinary Laboratories (DVL) Inc., based in Winnipeg, Manitoba. The company is Western Canada's largest manufacturer and distributor of veterinary pharmaceuticals, and describes itself as a family business. DVL has export ties to Trinidad, the Middle East and South Korea and was a 1999 Canada Export Award recipient.

Calcium supplements are salts of calcium used in a number of conditions. Supplementation is generally only required when there is not enough calcium in the diet. By mouth they are used to treat and prevent low blood calcium, osteoporosis, and rickets. By injection into a vein they are used for low blood calcium that is resulting in muscle spasms and for high blood potassium or magnesium toxicity.
Oral health can be difficult for pet owners and veterinary teams to manage in cats, particularly for pets whose owners are not committed to regular tooth brushing and/or dental treats. Oral disease is common among cats, and may lead to other health issues such as bacterial infections of major organs including the heart, kidneys and liver. When pet owners are aware of the benefits of supporting good oral health in cats, this substantially improves positive outcomes. Dietary selection, along with at-home-dental-hygiene care, allows cat owners to influence the oral status of their pets.