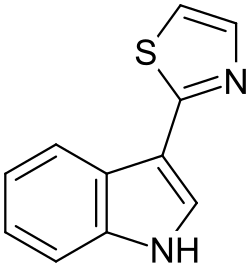
 | |
| Names | |
|---|---|
| Preferred IUPAC name 3-(1,3-Thiazol-2-yl)-1H-indole | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.236.489 |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C11H8N2S | |
| Molar mass | 200.26 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Camalexin (3-thiazol-2-yl-indole) is a simple indole alkaloid found in the plant Arabidopsis thaliana and other crucifers. The secondary metabolite functions as a phytoalexin to deter bacterial and fungal pathogens. [1]