
Secondary metabolites, also called specialised metabolites or secondary products, are a type of natural product generated by lifeforms that are not directly involved in the normal growth, development, or reproduction of the organism. Instead, they generally mediate ecological interactions, which may produce a selective advantage for the organism by increasing its survivability or fecundity. Specific secondary metabolites are often restricted to a narrow set of species within a phylogenetic group. Secondary metabolites often play an important role in plant defense against herbivory and other interspecies defenses. Humans use secondary metabolites as medicines, flavourings, pigments, and recreational drugs.

Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general phytoalexins are broad spectrum inhibitors; they are chemically diverse, and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids, and alkaloids; however, the term applies to any phytochemicals that are induced by microbial infection.
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

Flavoproteins are proteins that contain a nucleic acid derivative of riboflavin. These proteins are involved in a wide array of biological processes, including removal of radicals contributing to oxidative stress, photosynthesis, and DNA repair. The flavoproteins are some of the most-studied families of enzymes.
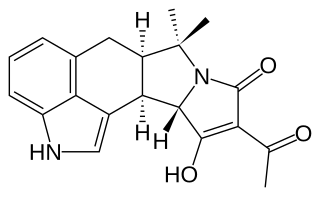
Cyclopiazonic acid (α-CPA), a mycotoxin and a fungal neurotoxin, is made by the molds Aspergillus and Penicillium. It is an indole-tetramic acid that serves as a toxin due to its ability to inhibit calcium-dependent ATPases found in the endoplasmic and sarcoplasmic reticulum. This inhibition disrupts the muscle contraction-relaxation cycle and the calcium gradient that is maintained for proper cellular activity in cells.
Polyketide synthases (PKSs) are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages. The biosyntheses of polyketides share striking similarities with fatty acid biosynthesis.

Cercospora is a genus of ascomycete fungi. Most species have no known sexual stage, and when the sexual stage is identified, it is in the genus Mycosphaerella. Most species of this genus cause plant diseases, and form leaf spots. It is a relatively well-studied genus of fungi, but there are countless species not yet described, and there is still much to learn about the best-known members of the genus.

Cercospora melongenae is a fungal plant pathogen that causes leaf spot on eggplant. It is a deuteromycete fungus that is primarily confined to eggplant species. Some other host species are Solanum aethiopicum and Solanum incanum. This plant pathogen only attacks leaves of eggplants and not the fruit. It is fairly common among the fungi that infect community gardens and home gardens of eggplant. Generally speaking, Cercospora melongenae attacks all local varieties of eggplants, but is most severe on the Philippine eggplant and less parasitic on a Siamese variety.
Shiraia bambusicola is a parasitic fungus on twigs of several genera of bamboos, and its relatively large stromata are used in traditional Chinese medicine. It is the sole species in the monotypic genus Shiraia. It is widely distributed in many provinces of Southern China and also in Japan.

A fungus is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as one of the traditional eukaryotic kingdoms, along with Animalia, Plantae and either Protista or Protozoa and Chromista.
Chaetomium cupreum is a fungus in the family Chaetomiaceae. It is able to decay in manufactured cellulosic materials, and is known to antagonize a wide range of soil microorganisms. This species is component of the biocontrol agent, Ketomium, a commercial biofungicide. It has also been investigated for use in the production of natural dyes. Chaetomium cupreum is mesophilic and known to occur in harsh environments and can rapidly colonize organic substrates in soil. Laboratory cultures of C. cupreum can be propagated on a range of common growth media including potato dextrose at ambient or higher than ambient temperature producing cottony white colonies with a reddish reverse.
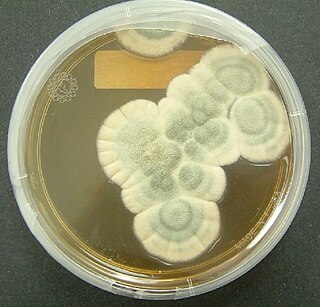
Penicillium chrysogenum is a species of fungus in the genus Penicillium. It is common in temperate and subtropical regions and can be found on salted food products, but it is mostly found in indoor environments, especially in damp or water-damaged buildings. It has been recognised as a species complex that includes P. notatum, P. meleagrinum, and P. cyaneofulvum. Molecular phylogeny has established that Alexander Fleming's first discovered penicillin producing strain is of a distinct species, P. rubens, and not of P. notatum. It has rarely been reported as a cause of human disease. It is the source of several β-lactam antibiotics, most significantly penicillin. Other secondary metabolites of P. chrysogenum include roquefortine C, meleagrin, chrysogine, 6-MSA YWA1/melanin, andrastatin A, fungisporin, secalonic acids, sorbicillin, and PR-toxin.

Grey leaf spot (GLS) is a foliar fungal disease that affects maize, also known as corn. GLS is considered one of the most significant yield-limiting diseases of corn worldwide. There are two fungal pathogens that cause GLS: Cercospora zeae-maydis and Cercospora zeina. Symptoms seen on corn include leaf lesions, discoloration (chlorosis), and foliar blight. Distinct symptoms of GLS are rectangular, brown to gray necrotic lesions that run parallel to the leaf, spanning the spaces between the secondary leaf veins. The fungus survives in the debris of topsoil and infects healthy crops via asexual spores called conidia. Environmental conditions that best suit infection and growth include moist, humid, and warm climates. Poor airflow, low sunlight, overcrowding, improper soil nutrient and irrigation management, and poor soil drainage can all contribute to the propagation of the disease. Management techniques include crop resistance, crop rotation, residue management, use of fungicides, and weed control. The purpose of disease management is to prevent the amount of secondary disease cycles as well as to protect leaf area from damage prior to grain formation. Corn grey leaf spot is an important disease of corn production in the United States, economically significant throughout the Midwest and Mid-Atlantic regions. However, it is also prevalent in Africa, Central America, China, Europe, India, Mexico, the Philippines, northern South America, and Southeast Asia. The teleomorph of Cercospora zeae-maydis is assumed to be Mycosphaerella sp.
Phenolic lipids are a class of natural products composed of long aliphatic chains and phenolic rings. Phenolic lipids occur in plants, fungi and bacteria.

C-5 sterol desaturase is an enzyme that is highly conserved among eukaryotes and catalyzes the dehydrogenation of a C-5(6) bond in a sterol intermediate compound as a step in the biosynthesis of major sterols. The precise structure of the enzyme's substrate varies by species. For example, the human C-5 sterol desaturase oxidizes lathosterol, while its ortholog ERG3 in the yeast Saccharomyces cerevisiae oxidizes episterol.
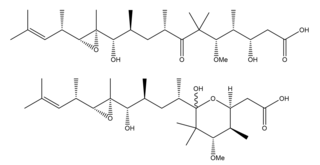
Gephyronic acid is a polyketide that exists as an equilibrating mixture of structural isomers. In nature, gephyronic acid is produced by slow growing myxobacterium: Archangium gephyra strain Ar3895 and Cystobacter violaceus strain Cb vi76. It is the first antibiotic in myxobacteria that was reported to specifically inhibit eukaryotic protein synthesis.

Lolitrem B is one of many toxins produced by a fungus called Epichloë festucae var. lolii), which grows in Lolium perenne. The fungus is symbiotic with the ryegrass; it doesn't harm the plant, and the toxins it produces kill insects that feed on ryegrass. Lolitrem B is one of these toxins, but it is also harmful to mammals. The shoots and flowers of infected ryegrass have especially high concentrations of lolitrem B, and when livestock eat too much of them, they get perennial ryegrass staggers. At low doses the animals have tremors, and at higher doses they stagger, and at higher yet doses the animals become paralyzed and die. The blood pressure of the animals also goes up. The effect of the lolitrem B comes on slowly and fades out slowly, as it is stored in fat after the ryegrass is eaten. The condition is especially common in New Zealand and Australia, and plant breeders there have been trying to develop strains of fungus that produce toxins only harmful to pests, and not to mammals.

BY1 is a taxonomically unidentified basidiomycete fungus. ITS sequencing has placed it in the Russulales and is referred to as a stereaceous basidiomycete. Chemotaxonomically supporting its placement in this group, it produces fomannoxins and vibralactones. The fungus' mycelia were isolated from dead aspen in Minnesota, USA. It is presumed to decompose wood by white rot.

Dihydromaltophilin, or heat stable anti-fungal factor (HSAF), is a secondary metabolite of Streptomyces sp. and Lysobacter enzymogenes. HSAF is a polycyclic tetramate lactam containing a single tetramic acid unit and a 5,5,6-tricyclic system. HSAF has been shown to have anti-fungal activity mediated through the disruption of the biosynthesis of Sphingolipid's by targeting a ceramide synthase unique to fungi.















