Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and beta-umbelliferone, is a natural product of the coumarin family.
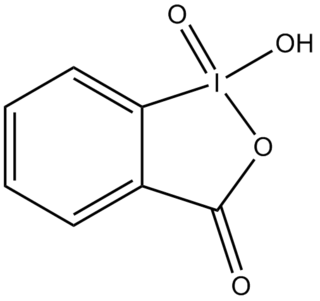
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is prepared from 2-iodobenzoic acid, potassium bromate, and sulfuric acid. Frigerio and co-workers have also demonstrated, in 1999 that potassium bromate may be replaced by commercially available Oxone. One of the main drawbacks of IBX is its limited solubility; IBX is insoluble in many common organic solvents. In the past, it was believed that IBX was shock sensitive, but it was later proposed that samples of IBX were shock sensitive due to the residual potassium bromate left from its preparation. Commercial IBX is stabilized by carboxylic acids such as benzoic acid and isophthalic acid.
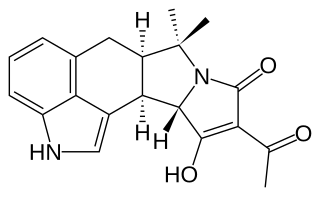
Cyclopiazonic acid (α-CPA), a mycotoxin and a fungal neurotoxin, is made by the molds Aspergillus and Penicillium. It is an indole-tetramic acid that serves as a toxin due to its ability to inhibit calcium-dependent ATPases found in the endoplasmic and sarcoplasmic reticulum. This inhibition disrupts the muscle contraction-relaxation cycle and the calcium gradient that is maintained for proper cellular activity in cells.

Epothilones are a class of potential cancer drugs. Like taxanes, they prevent cancer cells from dividing by interfering with tubulin, but in early trials, epothilones have better efficacy and milder adverse effects than taxanes.
The Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product. The mechanism of the reaction was proposed in its original disclosure by A.G. Brook with further evidence later supplied by George M. Rubottom. After a Prilezhaev-type oxidation of the silyl enol ether with the peroxyacid to form the siloxy oxirane intermediate, acid-catalyzed ring-opening yields an oxocarbenium ion. This intermediate then participates in a 1,4-silyl migration to give an α-siloxy carbonyl derivative that can be readily converted to the α-hydroxy carbonyl compound in the presence of acid, base, or a fluoride source.

Isomigrastatin is an analogue of migrastatin, an organic compound that naturally occurs in the Streptomyces platensis bacteria. Isomigrastatin has shown promise as a drug in the treatment of cancer. A laboratory synthesis was reported in 2007.

Pancratistatin (PST) is a natural compound initially extracted from spider lily, a Hawaiian native plant of the family Amaryllidaceae (AMD).
Streptogramin A is a group of antibiotics within the larger family of antibiotics known as streptogramins. They are synthesized by the bacteria Streptomyces virginiae. The streptogramin family of antibiotics consists of two distinct groups: group A antibiotics contain a 23-membered unsaturated ring with lactone and peptide bonds while group B antibiotics are depsipeptides. While structurally different, these two groups of antibiotics act synergistically, providing greater antibiotic activity than the combined activity of the separate components. These antibiotics have until recently been commercially manufactured as feed additives in agriculture, although today there is increased interest in their ability to combat antibiotic-resistant bacteria, particularly vancomycin-resistant bacteria.

Absinthin is a naturally produced triterpene lactone from the plant Artemisia absinthium (Wormwood). It constitutes one of the most bitter chemical agents responsible for absinthe's distinct taste. The compound shows biological activity and has shown promise as an anti-inflammatory agent, and should not to be confused with thujone, a neurotoxin also found in Artemisia absinthium.

An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon. In their largest application, oxaziridines are intermediates in the industrial production of hydrazine. Oxaziridine derivatives are also used as specialized reagents in organic chemistry for a variety of oxidations, including alpha hydroxylation of enolates, epoxidation and aziridination of olefins, and other heteroatom transfer reactions. Oxaziridines also serve as precursors to amides and participate in [3+2] cycloadditions with various heterocumulenes to form substituted five-membered heterocycles. Chiral oxaziridine derivatives effect asymmetric oxygen transfer to prochiral enolates as well as other substrates. Some oxaziridines also have the property of a high barrier to inversion of the nitrogen, allowing for the possibility of chirality at the nitrogen center.
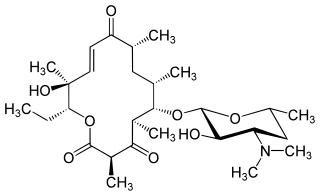
Pikromycin was studied by Brokmann and Hekel in 1951 and was the first antibiotic macrolide to be isolated. Pikromycin is synthesized through a type I polyketide synthase system in Streptomyces venezuelae, a species of Gram-positive bacterium in the genus Streptomyces. Pikromycin is derived from narbonolide, a 14-membered ring macrolide. Along with the narbonolide backbone, pikromycin includes a desosamine sugar and a hydroxyl group. Although Pikromycin is not a clinically useful antibiotic, it can be used as a raw material to synthesize antibiotic ketolide compounds such as ertythromycins and new epothilones.

Methylcyclohexene refers to any one of three organic compounds consisting of cyclohexene with a methyl group substituent. The location of the methyl group relative to the cyclohexene double bond creates the three different structural isomers. These compounds are generally used as a reagent or intermediate to derive other organic compounds.

Fumitremorgins are tremorogenic metabolites of Aspergillus and Penicillium, that belong to a class of naturally occurring 2,5-diketopiperazines.

Hectochlorin is a lipopeptide that exhibits potent antifungal activity against C. albicans and a number of plants pathogens, as well as inhibiting growth of human cell lines by hyperpolymerization of actin. It was originally isolated from the filamentous cyanobacterium Moorea producens JHB, collected from Hector Bay, Jamaica, 1996, which is a strain also known for being the producer of other two potent biomolecules named Jamaicamide A and Cryptomaldamide. Due to its activity against plants pathogens, synthetic efforts elucidated the compound’s total synthesis in 2002. Moorea species are normally the main component of the dietary of some sea hares, which concentrate the cyanobacterial metabolites as a mechanism of defense from predators. Therefore, in 2005, hectochlorin was re-isolated from the Thai sea hare Bursatella leachii, along with a new analogue, deacetylhectochlorin. Another reisolation of hectochlorin was reported in 2013, from another Moorea producens strain (RS05), isolated from the Red Sea, surprising in a non-tropical environment, as opposed to the other Moorea strains isolated before. The predicted biosynthesis of hectochlorin was published in 2007 and consists in a hybrid NRPS-PKS, with a hexanoic acid as start unit that becomes halogenated twice in the position 5, producing fairly rare gem-dichloro group, that along with two 2,3-dihydroxyisovaleric acid (DHIV) units compose a very interesting bioactive molecule.

Tirandamycins are a small group of natural products that contain a bicyclic ketal system and a tetramic acid moiety, the latter of which is found in different natural products from a variety of sources and which is characterized by a 2,4-pyrrolidinedione ring system. Members of this structural family have shown a wide range of biological activities like in antiparasitic, antifungal and anti-HIV evaluations, and furthermore, have shown potential usefulness because of their potent antibacterial properties. Streptolydigin, an analogue of the tirandamycins, is known to function as an antibacterial agent through inhibiting the chain initiation and elongation steps RNA polymerase transcription. The structural diversity in the tirandamycin family originates from the different oxidation patterns observed in the bicycic ketal system, and these modifications are determinant features for the bioactivity associated with these molecules.

Pseurotin A is a secondary metabolite of Aspergillus.
Fungal isolates have been researched for decades. Because fungi often exist in thin mycelial monolayers, with no protective shell, immune system, and limited mobility, they have developed the ability to synthesize a variety of unusual compounds for survival. Researchers have discovered fungal isolates with anticancer, antimicrobial, immunomodulatory, and other bio-active properties. The first statins, β-Lactam antibiotics, as well as a few important antifungals, were discovered in fungi.
Cytochrome P450 omega hydroxylases, also termed cytochrome P450 ω-hydroxylases, CYP450 omega hydroxylases, CYP450 ω-hydroxylases, CYP omega hydroxylase, CYP ω-hydroxylases, fatty acid omega hydroxylases, cytochrome P450 monooxygenases, and fatty acid monooxygenases, are a set of cytochrome P450-containing enzymes that catalyze the addition of a hydroxyl residue to a fatty acid substrate. The CYP omega hydroxylases are often referred to as monoxygenases; however, the monooxygenases are CYP450 enzymes that add a hydroxyl group to a wide range of xenobiotic and naturally occurring endobiotic substrates, most of which are not fatty acids. The CYP450 omega hydroxylases are accordingly better viewed as a subset of monooxygenases that have the ability to hydroxylate fatty acids. While once regarded as functioning mainly in the catabolism of dietary fatty acids, the omega oxygenases are now considered critical in the production or break-down of fatty acid-derived mediators which are made by cells and act within their cells of origin as autocrine signaling agents or on nearby cells as paracrine signaling agents to regulate various functions such as blood pressure control and inflammation.
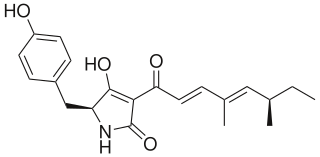
Pretenellin A is a secondary metabolite in Aspergillus oryzae. Pretenellin A is a substrate for tenellin because it undergoes an oxidative ring expansion to form Pretenellin B followed by N-hydroxylation to form Tenellin, an iron chelator in entomopathegnic fungus.

















