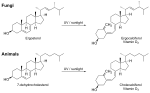
| Isolate | Source | Researched activity / Chemical description |
|---|
| 9-Deacetoxyfumigaclavine C | endophytic Aspergillus fumigatus | potent, selective, anticancer activity comparable to doxorubicin (IC50 = 3.1 μM against K562) [1] |
|---|
| 14-Norpseurotin A | Aspergillus | antiparasitic/anticancer [1] |
|---|
| 3-O-Methylfunicone | Penicillium pinophilum | in vitro cancer stem cell inhibitor |
|---|
| Anicequol | Penicillium aurantiogriseum | in vitro anchorage-independent cancer inhibitor |
|---|
| Anomalin A | sponge-derived Arthrinium | angiogenesis inhibitor |
|---|
| Antiamoebin | Emericellopsis | anti-microbial/protozoan polypeptide |
|---|
| Arugosin C | Aspergillus versicolor isolated from Red Sea green alga | bio-active anthraquinone [2] |
|---|
| Aspergillides A-C | marine Aspergillus ostianus | anticancer/cytotoxic |
|---|
| Aspergillusene | "sea fan"-derived Aspergillus sydowii | antioxidant sesquiterpene |
|---|
| Aspergilone A | "sea fan"-derived Aspergillus | anticancer and antifouling activity [3] |
|---|
| Aspergillusol A | marine Aspergillus | alpha-glucosidase inhibitor |
|---|
| Asperterrestide A | marine Aspergillus terreus | cytotoxic and antiviral cyclic tetrapeptide |
|---|
| Asterric acid | Antarctic Geomyces | endothelin binding inhibitor |
|---|
| Auranthine | Penicillium | antimicrobial |
|---|
| Aurantiamine | Penicillium aurantiogriseum | valine and histidine derived diketopiperazine |
|---|
| Aurantiomide | sponge-derived Penicillium aurantiogriseum | quinazoline alkaloid with cytotoxic/anticancer activity |
|---|
| Berkeleydione | fungal extremophile (Berkeley Pit, Montana) | anticancer polyketide-terpenoid |
|---|
| Berkeleytrione | fungal extremophile (Berkeley Pit, Montana) | anticancer polyketide-terpenoid |
|---|
| Berkelic acid | fungal extremophile (Berkeley Pit, Montana) | spiroketal anticancer compound |
|---|
| beta-Ergocryptine | ergot | dopaminergic ergot alkaloid |
|---|
| Bisvertinolone | Trichoderma | anticancer [4] |
|---|
| Botryodiplodin | Penicillium | antibiotic mycotoxin |
|---|
| Botryosphaeran | Botryosphaeria rhodina | free-radical scavenging and antioxidant [5] |
|---|
| Brevianamide S | marine Aspergillus versicolor | antimicrobial dimeric diketopiperazine |
|---|
| Brevicompanines D-H | deep ocean sediment Penicillium | lipopolysaccharide (LPS)-induced nitric oxide inhibitor |
|---|
| Cephalosporolide | marine Penicillium | novel lactones |
|---|
| Chaetoglobosin A | Chaetomium | anticancer [6] |
|---|
| Chaetoxanthone | marine-derived Chaetomium | bio-active xanthone |
|---|
| Chanoclavine | ergot | dopamine agonist |
|---|
| Chanoclavine II | ergot | |
|---|
| Chetracins B | Antarctic psychrophilic Oidiodendron truncatum | in vitro anticancer (nanomolar) |
|---|
| Chrysophanic acid | | antiviral/anticancer anthraquinone |
|---|
| Chrysosporide | Sepedonium chrysospermum | |
|---|
| Citreorosein | Penicillium | antimicrobial polyketide |
|---|
| Citrinolactone D | marine-derived Penicillium | citrinin derivative |
|---|
| Citromycetin | Australian Penicillium | bio-active polyketide |
|---|
| Citromycin | Penicillium | antibiotic |
|---|
| Communesin B | Mediterranean Axinella -derived Penicillium | anticancer |
|---|
| Costaclavin | ergot | |
|---|
| Cryptoechinuline D | mangrove rhizosphere soil-derived Aspergillus | anticancer |
|---|
| Curvularin | Penicillium | antimicrobial |
|---|
| Cycloprop-2-ene carboxylic acid | Russula subnigricans | causes rhabdomyolysis [7] |
|---|
| Decumbenone C | marine Aspergillus sulphureus | anticancer |
|---|
| Dehydroaltenusin | Alternaria tenuis | inhibitor of mammalian DNA polymerase α |
|---|
| Dehydrocurvularin | Penicillium | antimicrobial |
|---|
| Disydonols A-C | marine Aspergillus | anticancer |
|---|
| Duclauxin | Penicillium duclauxi | anticancer [8] |
|---|
| Epicoccins | Cordyceps -colonizing Epicoccum nigrum | antiviral |
|---|
| Epolactaene | marine fungus | antiinflammatory, inhibitory activity of DNA polymerases and DNA topoisomerase II, active synthetic analogs [9] |
|---|
| Epoxyagroclavine | permafrost Penicillium | ergot alkaloid |
|---|
| Epoxyphomalins A-B | marine Paraconiothyrium | potent cytotoxics |
|---|
| Ergosine | ergot | dopaminergic ergot alkaloid |
|---|
| Ergostane | mushrooms | steroid |
|---|
| Ergostine | ergot | alpha-adrenergic blocking, vasoconstrictive ergot alkaloid |
|---|
| Eupenifeldin | Eupenicillium brefeldianum | antimicrobial cytotoxic bistropolone |
|---|
| Evariquinone | Emericella variecolor (derived from the marine sponge Haliclona ) | |
|---|
| Fecosterol | fungi and lichens | steroid |
|---|
| Fellutanine | Penicillium | bio-active diketopiperazine alkaloids |
|---|
| Festuclavine | Aspergillus fumigatus | bio-active ergoline |
|---|
| Fumigaclavine A | endophytic Aspergillus | bio-active ergoline |
|---|
| Fumigaclavine B | endophytic Aspergillus | bio-active ergoline |
|---|
| Fumigaclavine C | endophytic Aspergillus | bio-active ergoline |
|---|
| Fumiquinazoline | soft coral Sinularia -derived Aspergillus fumigatus | cytotoxic/anticancer |
|---|
| Fungisterol | Cordyceps sinensis | steroid |
|---|
| Glionitrin A | mine-dwelling Aspergillus fumigatus | antibiotic-anticancer |
|---|
| Glionitrin B | Aspergillus fumigatus KMC-901 | anticancer diketopiperazine |
|---|
| Hymenosetin | Hymenoscyphus pseudoalbidus | antimicrobial (active against MRSA) [10] |
|---|
| Integrasone | Unknown | inhibits HIV-1 integrase enzyme [11] |
|---|
| Isoemericellin | marine Emericella variecolor | |
|---|
| Leporizines A-C | Aspergillus | cytotoxic epithiodiketopiperazines |
|---|
| Leptosphaerin | marine Leptosphaeria oraemaris | antifungal |
|---|
| Lichesterol | fungi and lichens | steroid |
|---|
| Luteoalbusins A-B | deep sea Acrostalagmus luteoalbus | anticancer indole diketopiperazines |
|---|
| Luteusin A | Talaromyces luteus | monoamine oxidase inhibitor |
|---|
| Malettinin | Hypoxylon | polyketide/antimicrobial |
|---|
| Maximiscin | Tolypocladium (Salcha, Alaska) [12] | anticancer polyketide-shikimate compound |
|---|
| Meleagrin | deep ocean Penicillium | anticancer |
|---|
| Methylenolactocin | Penicillium | anticancer |
|---|
| Neoxaline | Aspergillus japonicus | antimitotic and antiplatelet |
|---|
| Nigerapyrones A-E | marine mangrove-derived, endophytic Aspergillus niger | anticancer |
|---|
| Nigrosporin B | Nigrospora | antimicrobial |
|---|
| Nocapyrones E-G | Nocardiopsis dassonvillei | antimicrobial alpha-pyrones |
|---|
| Notoamide | marine Aspergillus | bio-active prenylated indole alkaloid |
|---|
| Oxaline | Penicillium oxalicum and Aspergillus japonicus | anticancer (tubulin polymerization inhibitor), O-methylated derivative of meleagrin |
|---|
| Pencolide | seaweed-derived endophytic fungi | bio-active maleimide |
|---|
| Penicitrinol J | marine-derived Penicillium | bio-active citrinin dimer |
|---|
| Penicitrinol K | marine-derived Penicillium | bio-active citrinin derivative |
|---|
| Penicitrinone E | marine-derived Penicillium | bio-active citrinin dimer |
|---|
| Penochalasin A | endophytic Chaetomium | cytotoxic/anticancer cytochalasan-based alkaloid |
|---|
| Penostatin A | Penicillium | cytotoxic metabolite |
|---|
| Pestalamides A-C | Pestalotiopsis theae | antiviral and antifungal |
|---|
| Petrosifungin | sponge-derived Penicillium brevicompactum | novel cyclodepsipeptide |
|---|
| Phillyrin | endophytic fungus (isolated from Forsythia ) | antiobesity |
|---|
| Piscarinine | Penicillium piscarium westling | bio-active polycyclic diketopiperazine alkaloid |
|---|
| Prenylterphenyllins | marine Aspergillus candidus | anticancer |
|---|
| Protuboxepins A and B | Aspergillus SF-5044 | anticancer diketopiperazines |
|---|
| Pseurotin A | endophytic Aspergillus | antiparasitic and anticancer |
|---|
| Pyrenocine | marine Penicillium paxilli | antibiotic/antiinflammatory mycotoxin [13] |
|---|
| Questiomycin A | Penicillium expansum | antibiotic |
|---|
| Quinocitrinine | permafrost Penicillium | quinoline alkaloid |
|---|
| RES-1149-2 | Aspergillus | non-peptidic endothelin receptor antagonist |
|---|
| Retigeric acid B | Lobaria (lichen) | anticancer |
|---|
| Rubratoxin B | Penicillium rubrum | anticancer |
|---|
| Rugulovasine | Penicillium | |
|---|
| Sch 642305 | Penicillium verrucosum and Rhizoctonia solani | bacterial DNA primase inhibitor |
|---|
| Sclerotides A-B | Aspergillus sclerotiorum PT06-1 | bio-active cyclic hexapeptides |
|---|
| Secalonic acid | marine fungi | nootropic |
|---|
| Shamixanthone | Aspergillus | bio-active prenylated xanthone |
|---|
| Shearinine | marine Penicillium janthinellum | anticancer |
|---|
| Siderin | Aspergillus versicolor isolated from Red Sea green alga | bio-active anthraquinone |
|---|
| Sorbicillactone A | sponge-derived fungus | novel bio-active alkaloid |
|---|
| Spiculisporic acid | marine Aspergillus | bioactive γ-butenolide |
|---|
| Spiropreussione | Preussia | anticancer |
|---|
| Stephacidin | Aspergillus ochraceus WC76466 | anticancer/cytotoxic |
|---|
| Stromemycin | marine Emericella | C-glycosidic depside matrix metalloproteinase inhibitor |
|---|
| Terpestacin | endophytic fungus Drechslera ravenelii | anticancer |
|---|
| Terrestrols | marine Penicillium terrestre | cytotoxic/anticancer [14] |
|---|
| Terreulactone A | Aspergillus terreus | anti-acetylcholinesterase terpenoid |
|---|
| Topopyrone C | Phoma and Penicillium | anticancer human topoisomerase I inhibitor |
|---|
| Trachyspic acid | Talaromyces trachyspermus | heparanase inhibitor |
|---|
| Trichodimerol | Trichoderma | bio-active pentacycle |
|---|
| Ustusolates | marine Aspergillus ustus | anticancer |
|---|
| Variecolactone | Emericella purpurea mycelium | immunomodulatory sesterterpene |
|---|
| Variecolol | Emericella aurantio-brunnea | immunosuppressant/antiviral alkaloid |
|---|
| Varixanthone | marine Emericella variecolor | antimicrobial |
|---|
| Vermiculine | Penicillium vermiculatum | antibiotic |
|---|
| Vermistatin | fungal extremophile (Berkeley Pit, Montana) | anticancer [15] |
|---|
| Vermixocin | Penicillium vermiculatum | cytotoxic metabolite |
|---|
| Verrucosidin | Penicillium verrucosum | cytotoxic pyrone-type polyketide |
|---|
| Verrulactone A | Penicillium | antimicrobial alternariol |
|---|
| Versicolamide B | marine Aspergillus | a paraherquamide-stephacidin |
|---|
| Viscumamide | mangrove-derived endophytic fungi | cyclic peptide |
|---|
| Yaequinolone J1 | Penicillium sp. FKI-2140 | antibiotic |
|---|
|