
Ergot or ergot fungi refers to a group of fungi of the genus Claviceps.

Ergotism is the effect of long-term ergot poisoning, traditionally due to the ingestion of the alkaloids produced by the Claviceps purpurea fungus—from the Latin noun clava meaning "club" and the suffix -ceps meaning "head", i.e. the purple club-headed fungus—that infects rye and other cereals, and more recently by the action of a number of ergoline-based drugs. It is also known as ergotoxicosis, ergot poisoning, and Saint Anthony's fire.

Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.
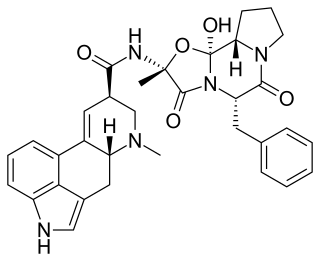
Ergotamine, sold under the brand names Cafergot and Ergomar among others, is an ergopeptine and part of the ergot family of alkaloids; it is structurally and biochemically closely related to ergoline. It possesses structural similarity to several neurotransmitters, and has biological activity as a vasoconstrictor.

D-Lysergic acid α-hydroxyethylamide, also known as D-lysergic acid methyl carbinolamide, is an alkaloid of the ergoline family, believed to be present in small amounts in various species in the Convolvulaceae, as well as some species of fungi.

Methylergometrine, also known as methylergonovine and sold under the brand name Methergine, is a medication of the ergoline and lysergamide groups which is used as an oxytocic in obstetrics and in the treatment of migraine. It reportedly produces psychedelic effects similar to those of lysergic acid diethylamide (LSD) at high doses.

Claviceps purpurea is an ergot fungus that grows on the ears of rye and related cereal and forage plants. Consumption of grains or seeds contaminated with the survival structure of this fungus, the ergot sclerotium, can cause ergotism in humans and other mammals. C. purpurea most commonly affects outcrossing species such as rye, as well as triticale, wheat and barley. It affects oats only rarely.

Ergocryptine is an ergopeptine and one of the ergot alkaloids. It is isolated from ergot or fermentation broth and it serves as starting material for the production of bromocriptine.
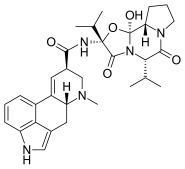
Ergocornine is a crystalline ergopeptine and one of the ergot alkaloids separated from ergotoxine. It is also a dopamine receptor agonist. It was discovered by Albert Hofmann, the Swiss chemist who created LSD.

Agroclavine belongs to the group of ergot alkaloids, such as ergotamine. Historically, the main use of agroclavine was to oxidize it to elymoclavine, which can then be used for ergot-based drug synthesis.
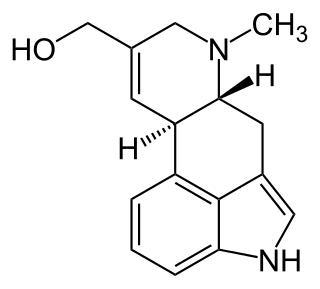
Elymoclavine is an ergot alkaloid. It can be produced from C. fusiformis from Pennisetum typhoideum. It is a precursor in the biosynthesis of D-(+)-lysergic acid. Ergot alkaloids are natural products derived from L-tryptophan. They are often toxic for humans and animals. Despite that they are also well known for their pharmacological activities.
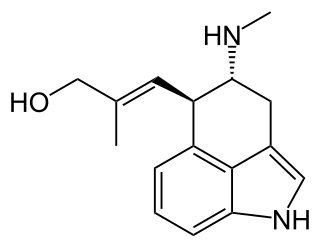
Chanoclavine, also known as chanoclavin-l is a tri-cyclic ergot alkaloid (ergoline) isolate of certain fungi. It is mainly produced by members of the genus claviceps. Long used in traditional Chinese medicine, it was found in 1987 mouse studies to stimulate dopamine D2 receptors in the brain.
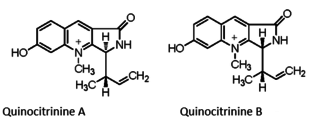
Quinocitrinines are quinoline alkaloids isolated from a permafrost Penicillium.
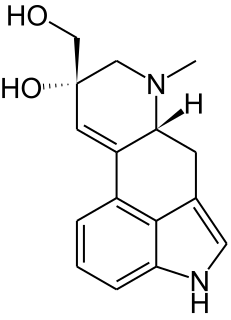
Penniclavine is an ergot alkaloid.

Setoclavine is an ergot alkaloid.
Medicinal fungi are fungi which contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.
Penicillium corylophilum is a species of the genus of Penicillium which occurs in damp buildings in United States, Canada and western Europe but it can also be found in a variety of foods and mosquitoes. Penicillium corylophilum produces the alkaloid epoxyagroclavine and citrinin and is a pathogen to mosquitoes.
Penicillium gorlenkoanum is a species of the genus of Penicillium which produces citrinin, costaclavine and epicostaclavine.
Penicillium palitans is an anamorph species of fungus in the genus Penicillium which was isolated from cheese and ancient permafrost deposits. Penicillium palitans produces viridicatin, cyclopiazonic acid, roquefortine, palitantin and ochratoxin A
Penicillium sizovae is an anamorph species of fungus in the genus Penicillium which produces agroclavine-I and epoxyagroclavine-I.















