Dipeptidyl peptidase-4 (DPP4), also known as adenosine deaminase complexing protein 2 or CD26 is a protein that, in humans, is encoded by the DPP4 gene. DPP4 is related to FAP, DPP8, and DPP9. The enzyme was discovered in 1966 by Hopsu-Havu and Glenner, and as a result of various studies on chemism, was called dipeptidyl peptidase IV [DP IV].

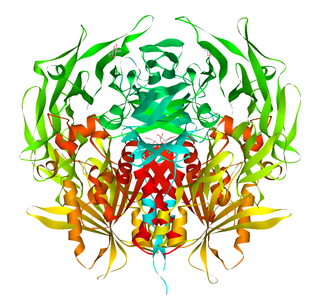
Prolyl endopeptidase (PE) also known as prolyl oligopeptidase or post-proline cleaving enzyme is an enzyme that in humans is encoded by the PREP gene.

Staurosporine is a natural product originally isolated in 1977 from the bacterium Streptomyces staurosporeus. It was the first of over 50 alkaloids to be isolated with this type of bis-indole chemical structure. The chemical structure of staurosporine was elucidated by X-ray analysis of a single crystal and the absolute stereochemical configuration by the same method in 1994.
Penicillium aurantiogriseum is a plant pathogen infecting asparagus and strawberry. Chemical compounds isolated from Penicillium aurantiogriseum include anicequol and auranthine.

Procollagen-proline dioxygenase, commonly known as prolyl hydroxylase, is a member of the class of enzymes known as alpha-ketoglutarate-dependent hydroxylases. These enzymes catalyze the incorporation of oxygen into organic substrates through a mechanism that requires alpha-Ketoglutaric acid, Fe2+, and ascorbate. This particular enzyme catalyzes the formation of (2S, 4R)-4-hydroxyproline, a compound that represents the most prevalent post-translational modification in the human proteome.

Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 is an enzyme that in humans is encoded by the PIN1 gene.

Hypoxia-inducible factor prolyl hydroxylase 2 (HIF-PH2), or prolyl hydroxylase domain-containing protein 2 (PHD2), is an enzyme encoded by the EGLN1 gene. It is also known as Egl nine homolog 1. PHD2 is a α-ketoglutarate/2-oxoglutarate-dependent hydroxylase, a superfamily non-haem iron-containing proteins. In humans, PHD2 is one of the three isoforms of hypoxia-inducible factor-proline dioxygenase, which is also known as HIF prolyl-hydroxylase.

Hydnellum is a genus of tooth fungi in the family Bankeraceae. Widely distributed in the Northern Hemisphere, the genus contains around 40 species. The fruitbodies of its members grow by slowly enveloping nearby bits of grass and vegetation. There is great variability in the form of Hydnellum fruitbodies, which are greatly influenced by environmental conditions such as rainfall and humidity, drying winds, and temperature. They are too tough and woody to eat comfortably. Several species have become the focus of increasing conservation concern following widespread declines in abundance.

Polyozellus is a fungal genus in the family Thelephoraceae, a grouping of mushrooms known collectively as the leathery earthfans. A monotypic genus, it contains the single species Polyozellus multiplex, first described in 1899, and commonly known as the blue chanterelle, the clustered blue chanterelle, or, in Alaska, the black chanterelle. The distinctive fruit body of this species comprises blue- to purple-colored clusters of vase- or spoon-shaped caps with veiny wrinkles on the undersurface that run down the length of the stem.

Thelephoric acid is a terphenylquinone pigment that is found in several fungi, such as Omphalotus subilludens and Polyozellus multiplex. Thelephoric acid has been shown to inhibit prolyl endopeptidase, an enzyme that has a role in processing proteins in Alzheimer's disease. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects. It is derived from atromentin, and its precursor can be from cyclovariegatin. Fragmentation patterns have suggested that polymers of thelephoric acid exists.
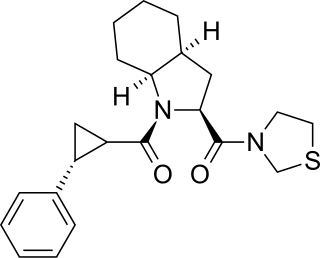
S-17092 is a drug which acts as a selective inhibitor of the enzyme prolyl endopeptidase. This enzyme is involved in the metabolic breakdown of a number of neuropeptide neurotransmitters in the brain, and so inhibiting the action of the enzyme increases the activity of these neuropeptides. This produces nootropic effects which make S-17092 a promising and novel treatment for neurodegenerative conditions such as Alzheimer's disease and Parkinson's disease.

Kynapcin is a general name for a number of dibenzofuranyl derivatives of the molecule polyozellin, present in the fungus Polyozellus multiplex. Like polyozellin, some kynapcins inhibit prolyl endopeptidase, an enzyme that has a role in processing proteins including amyloid precursor protein. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects. Several kynapcins have been found in P. multiplex, each with different chemical properties, including kynapcin-12, kynapcin-13 and -28, and -24. A total synthesis of kynapcin-24 was achieved in 2009.

Romidepsin, also known as Istodax, is an anticancer agent used in cutaneous T-cell lymphoma (CTCL) and other peripheral T-cell lymphomas (PTCLs). Romidepsin is a natural product obtained from the bacterium Chromobacterium violaceum, and works by blocking enzymes known as histone deacetylases, thus inducing apoptosis. It is sometimes referred to as depsipeptide, after the class of molecules to which it belongs. Romidepsin is branded and owned by Gloucester Pharmaceuticals, now a part of Celgene.
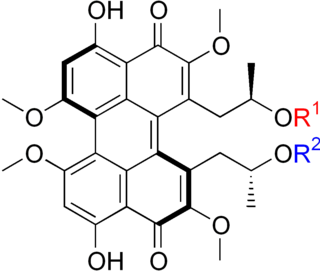
The calphostins are a class of closely related chemical compounds isolated from the fungus Cladosporium cladosporioides. The known calphostins include calphostin A, calphostin B, calphostin C, calphostin D, and calphostin I. The calphostins are inhibitors of protein kinase C (PKC). The most potent member of the series, calphostin C, has found use as a biochemical tool because of this activity.

Calphostin C is a natural chemical compound. It is one of the calphostins, isolated from the fungus Cladosporium cladosporioides. Calphostin C is a potent inhibitor of protein kinase C (PKC).

An Oligopeptidase is an enzyme that cleaves peptides but not proteins. This property is due to its structure: the active site of this enzyme is located at the end of a narrow cavity which can only be reached by peptides.
Fungal isolates have been researched for decades. Because fungi often exist in thin mycelial monolayers, with no protective shell, immune system, and limited mobility, they have developed the ability to synthesize a variety of unusual compounds for survival. Researchers have discovered fungal isolates with anticancer, antimicrobial, immunomodulatory, and other bio-active properties. The first statins, β-Lactam antibiotics, as well as a few important antifungals, were discovered in fungi.
Penicillium herquei is an anamorph, filamentous species of the genus of Penicillium which produces citreorosein, emodin, hualyzin, herquline B, janthinone, citrinin and duclauxin,.
Streptomyces kasugaensis is a bacterium species from the genus of Streptomyces which has been isolated from soil from the city Nara in Japan. Streptomyces kasugaensis produces kasugamycin and thiolutin.
Christopher Joseph Schofield is the Head of Organic Chemistry at the University of Oxford and a Fellow of the Royal Society. Chris Schofield is a professor of organic chemistry at the University of Oxford, Department of Chemistry and a Fellow of Hertford College. Prof Schofield studied functional, structural and mechanistic understanding of enzymes that employ oxygen and 2-oxoglutarate as a co-substrate. His work has opened up new possibilities in antibiotic research, oxygen sensing, and gene regulation.















