In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.

In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to an organyl group, or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (EC number 4.1.1).
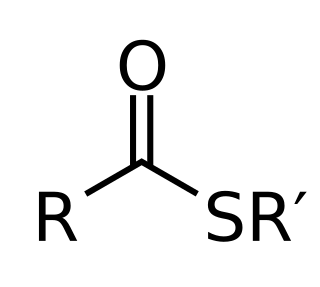
In organic chemistry, thioesters are organosulfur compounds with the molecular structure R−C(=O)−S−R’. They are analogous to carboxylate esters with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid with a thiol. In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA. The R and R' represent organyl groups, or H in the case of R.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

A dipeptide is an organic compound derived from two amino acids. The constituent amino acids can be the same or different. When different, two isomers of the dipeptide are possible, depending on the sequence. Several dipeptides are physiologically important, and some are both physiologically and commercially significant. A well known dipeptide is aspartame, an artificial sweetener.
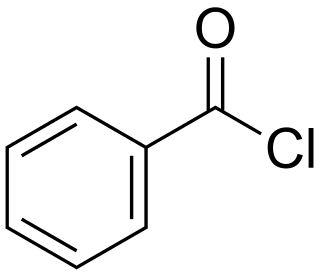
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C7H5ClO. It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring with an acyl chloride substituent. It is mainly useful for the production of peroxides but is generally useful in other areas such as in the preparation of dyes, perfumes, pharmaceuticals, and resins.

Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2(CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium.
The Ullmann condensation or Ullmann-type reaction is the copper-promoted conversion of aryl halides to aryl ethers, aryl thioethers, aryl nitriles, and aryl amines. These reactions are examples of cross-coupling reactions.

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.

Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste. The molecule consists of a benzene ring, ortho-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic functional groups, the compound is amphoteric. Anthranilic acid is a white solid when pure, although commercial samples may appear yellow. The anion [C6H4(NH2)(CO2)]−, obtained by the deprotonation of anthranilic acid, is called anthranilate. Anthranilic acid was once thought to be a vitamin and was referred to as vitamin L1 in that context, but it is now known to be non-essential in human nutrition.
Pivalic acid is a carboxylic acid with a molecular formula of (CH3)3CCO2H. This colourless, odiferous organic compound is solid at room temperature. Two abbreviations for pivalic acid are t-BuC(O)OH and PivOH. The pivalyl or pivaloyl group is abbreviated t-BuC(O).

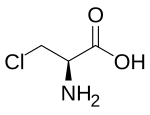
2-Chloropropionic acid (2-chloropropanoic acid) is the chemical compound with the formula CH3CHClCO2H. This colorless liquid is the simplest chiral chlorocarboxylic acid, and it is noteworthy for being readily available as a single enantiomer. The conjugate base of 2-chloropropionic acid (CH3CHClCO2−), as well as its salts and esters, are known as 2-chloropropionates or 2-chloropropanoates.

Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. It is a yellow solid that is soluble in some organic solvents. It is used for palladium-catalyzed coupling reactions, e.g. the Sonogashira–Hagihara reaction. The complex is square planar. Many analogous complexes are known with different phosphine ligands.

Aminomethyl propanol (AMP) is an organic compound with the formula H2NC(CH3)2CH2OH. It is colorless liquid that is classified as an alkanolamine. It is a useful buffer and a precursor to numerous other organic compounds.

tert-Butyl peroxybenzoate (TBPB) an organic compound with the formula C6H5CO3CMe3 (Me = CH3). It is the most widely produced perester; it is an ester of peroxybenzoic acid (C6H5CO3H). It is often used as a radical initiator in polymerization reactions, such as the production of LDPE from ethylene, and for crosslinking, such as for unsaturated polyester resins.

Phenylglycine is the organic compound with the formula C6H5CH(NH2)CO2H. It is a non-proteinogenic alpha amino acid related to alanine, but with a phenyl group in place of the methyl group. It is a white solid. The compound exhibits some biological activity.

Tris(o-tolyl)phosphine is an organophosphorus compound with the formula P(C6H4CH3)3. It is a white, water-insoluble solid that is soluble in organic solvents. In solution it slowly converts to the phosphine oxide. As a phosphine ligand, it has a wide cone angle of 194°. Consequently, it tends to cyclometalate when treated with metal halides and metal acetates. Complexes of this ligand are common in homogeneous catalysis.
Glycine methyl ester hydrochloride is the organic compound with the formula [CH3O2CCH2NH3]Cl. A white, water-soluble solid, it is the hydrochloride of the methyl ester of the amino acid glycine.