
Pseudevernia furfuracea, commonly known as tree moss, is a lichenized species of fungus that grows on the bark of firs and pines. The lichen is rather sensitive to air pollution, its presence usually indicating good air conditions in the growing place. The species has numerous human uses, including use in perfume, embalming and in medicine. Large amounts of tree moss is annually processed in France for the perfume industry.

A depside is a type of polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester group. Depsides are most often found in lichens, but have also been isolated from higher plants, including species of the Ericaceae, Lamiaceae, Papaveraceae and Myrtaceae.

Olivetol is an organic compound with the formula C5H11C6H3(OH)2. Several isomers exist; olivetol has the two hydroxyl and the pentyl groups located at the 1,3, and 5 positions on the benzene ring. It is a colorless solid that is soluble in a variety of organic solvents. It is found in certain species of lichen. It is also a precursor in various syntheses of tetrahydrocannabinol.

Parmotrema perlatum, commonly known as the powdered ruffle lichen, is a common species of foliose lichen in the family Parmeliaceae. The species has a cosmopolitan distribution and occurs throughout the Northern and Southern Hemispheres. Parmotrema perlatum is a prominent and widely recognised species within its genus across primarily temperate zones, preferring humid, oceanic-suboceanic habitats. It is found in diverse geographic areas including Africa, North and South America, Asia, Australasia, Europe, and islands in the Atlantic and Pacific oceans. It usually grows on bark, but occasionally occurs on siliceous rocks, often among mosses.
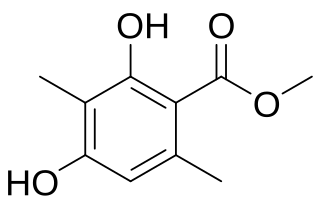
Atraric acid is a naturally occurring phenolic compound and ester with the IUPAC name methyl 2,4-dihydroxy-3,6-dimethylbenzoate and molecular formula C10H12O4. It occurs in the root-bark of Pygeum africanum and Evernia prunastri (Oakmoss). There is evidence to suggest that it has antiandrogenic activity in humans and its use in treatment of benign prostate hyperplasia, prostate cancer, and spinal and bulbar muscular atrophy has been investigated.

Atranorin is a chemical substance produced by some species of lichen. It is a secondary metabolite belonging to a group of compounds known as depsides. Atranorin has analgesic, anti-inflammatory, antibacterial, antifungal, cytotoxic, antioxidant, antiviral, and immunomodulatory properties. In rare cases, people can have an allergic reaction to atranorin.

Pleurosticta acetabulum is a species of foliose lichen in the family Parmeliaceae. It is common and widespread throughout Europe, where it grows on tree bark. It has also been recorded in Algeria.

Evernic acid is an organic compound and depside with the molecular formula C17H16O7. Evernic acid was first isolated from the lichen Usnea longissima. Evernic acid is soluble in hot alcohol and poorly soluble in water. Evernic acid is produced by the lichens Ramalina, Evernia, and Hypogymnia.
Punctelia diffractaica is a species of foliose lichen in the family Parmeliaceae. It is found in Peru.

Hypogymnia tubulosa is a species of foliose lichen in the family Parmeliaceae. Ludwig Emanuel Schaerer formally described it in 1840 as a variety of Parmelia ceratophylla. Johan Johnsen Havaas promoted it to distinct species status in 1918.

Salazinic acid is a depsidone with a lactone ring. It is found in some lichens, and is especially prevalent in Parmotrema and Bulbothrix, where its presence or absence is often used to help classify species in those genera.

Lichexanthone is an organic compound in the structural class of chemicals known as xanthones. Lichexanthone was first isolated and identified by Japanese chemists from a species of leafy lichen in the 1940s. The compound is known to occur in many lichens, and it is important in the taxonomy of species in several genera, such as Pertusaria and Pyxine. More than a dozen lichen species have a variation of the word lichexanthone incorporated as part of their binomial name. The presence of lichexanthone in lichens causes them to fluoresce a greenish-yellow colour under long-wavelength UV light; this feature is used to help identify some species. Lichexanthone is also found in several plants, and some species of fungi that do not form lichens.
Lichen products, also known as lichen substances, are organic compounds produced by a lichen. Specifically, they are secondary metabolites. Lichen products are represented in several different chemical classes, including terpenoids, orcinol derivatives, chromones, xanthones, depsides, and depsidones. Over 800 lichen products of known chemical structure have been reported in the scientific literature, and most of these compounds are exclusively found in lichens. Examples of lichen products include usnic acid, atranorin, lichexanthone, salazinic acid, and isolichenan, an α-glucan. Many lichen products have biological activity, and research into these effects is ongoing.

Barbatic acid is an organic compound that is made by some lichens. It is in the structural class known as depsides. It is particularly common in the genera Usnea and Cladonia.

Platismatia glauca is a common and widespread species of corticolous (bark-dwelling), foliose lichen in the family Parmeliaceae.

Parietinic acid is an organic compound in the structural class of chemicals known as anthraquinones. It is found in many species of the lichen family Teloschistaceae. The substance was first reported in the literature by the German chemist Walter Eschrich in 1958.

Fallacinol (teloschistin) is an organic compound in the structural class of chemicals known as anthraquinones. It is found in some lichens, particularly in the family Teloschistaceae, as well as a couple of plants and non lichen-forming fungi. In 1936, Japanese chemists isolated a pigment they named fallacin from the lichen Oxneria fallax, which was later refined and assigned a tentative structural formula; by 1949, Indian chemists had isolated a substance from Teloschistes flavicans with an identical structural formula to fallacin. Later research further separated fallacin into two distinct pigments, fallacin-A and fallacin-B (fallacinol). The latter compound is also known as teloschistin due to its structural match with the substance isolated earlier.

Confluentic acid is an organic compound belonging to the chemical class known as depsides. It serves as a secondary metabolite in certain lichens and plays a role in distinguishing closely related species within the genus Porpidia. In 1899, Friedrich Wilhelm Zopf isolated a compound from Lecidea confluens, which he initially named confluentin and noted for its melting point of 147–148 °C. This substance demonstrated the ability to turn litmus paper red and, when interacting with alkali, decomposed into carbon dioxide and phenol-like compounds. Zopf subsequently revised the chemical formula and melting point of the compound. Siegfried Huneck renamed it confluentinic acid in 1962, characterising it as optically inactive, with distinct colour reactions and solubility properties, and determined its molecular formula as C28H36O8.

Grayanic acid is an organic compound found in certain lichens, particularly Cladonia grayi, where it serves as a secondary metabolite with notable taxonomic importance. Identified in the 1930s, it is now recognised as a chemotaxonomic marker that helps distinguish closely related species within the Cladonia chlorophaea species group. Grayanic acid crystallises as colourless, needle-like structures, melts at approximately 186–189 °C (367–372 °F), and displays distinctive fluorescence under ultraviolet light, aiding in its detection and study.

Divaricatic acid is a chemical compound with the molecular formula C21H24O7. It is found in a variety of lichens, including some in the genera Evernia, and Lepraria, and Ramalina. It is classified as a depside.


















