
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

β-Carboline (9H-pyrido[3,4-b]indole), also known as norharmane, is a nitrogen containing heterocycle. It is also the prototype of a class of compounds known as β-carbolines.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure C6H5CH2–. Benzyl features a benzene ring attached to a CH2 group.

Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. Its corresponding conjugate acid at pH 7.3 major species is known as tropiniumone.
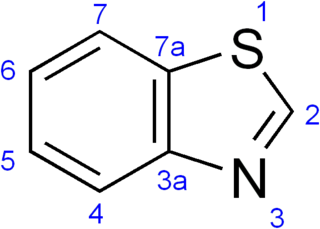
Benzothiazole is an aromatic heterocyclic compound with the chemical formula C
7H
5NS. It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole.

Huperzine A is a naturally occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata and in varying quantities in other Huperzia species, including H. elmeri, H. carinat, and H. aqualupian. Huperzine A has been investigated as a treatment for neurological conditions such as Alzheimer's disease, but a meta-analysis of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution. Huperzine A inhibits the breakdown of the neurotransmitter acetylcholine by the enzyme acetylcholinesterase. It is commonly available over the counter as a nutrient supplement, and is marketed as a cognitive enhancer for improving memory and concentration.

Aporphine is an alkaloid that forms the core of a class of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.

Choisya ternata is a species of flowering plant in the family Rutaceae, known as Mexican orange blossom or Mexican orange.

Lupinus angustifolius is a species of lupin known by many common names, including narrowleaf lupin, narrow-leaved lupin and blue lupin. It is native to Eurasia and northern Africa and naturalized in parts of Australia and North America. It has been cultivated for over 6000 years as a food crop for its edible legume seeds, as a fodder for livestock and for green manure.

Acacia complanata, known as long-pod wattle and flat-stemmed wattle, is a perennial tree native to Australia. It can grow 5–6 m tall, but more often it grows as a large shrub. It is not listed as being a threatened species. It is commonly used in environmental management.

8-Hydroxyquinoline is an organic compound with the formula C9H7NO. It is a derivative of the heterocycle quinoline by placement of an OH group on carbon number 8. This light yellow compound is widely used commercially, although under a variety of names.

The Gould–Jacobs reaction is an organic synthesis for the preparation of quinolines and 4‐hydroxyquinoline derivatives. The Gould-Jacobs reaction is a series of reactions. The series of reactions begins with the condensation/substitution of an aniline with alkoxy methylenemalonic ester or acyl malonic ester, producing anilidomethylenemalonic ester. Then through a 6 electron cyclization process, 4-hydroxy-3-carboalkoxyquinoline is formed, which exist mostly in the 4-oxo form. Saponification results in the formation of an acid. This step is followed by decarboxylation to give 4-hydroxyquinoline. The Gould-Jacobs reaction is effective for anilines with electron‐donating groups at the meta‐position.
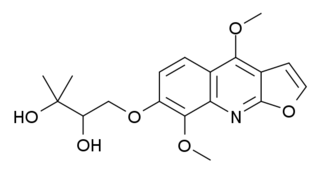
Evoxine (haploperine) is a furoquinoline alkaloid with hypnotic and sedative effects. It is found naturally in a variety of Australian and African plants including Evodia xanthoxyloides and Teclea gerrardii.

Nembrotha purpureolineata is a species of colourful sea slug, a dorid nudibranch, a marine gastropod mollusc in the family Polyceridae. Nembrotha rutilans, classified as a separate species until 2008, has now been reclassified as Nembrotha purpureolineata.
Dodonaea petiolaris is a shrub species in the genus Dodonaea found in Australia.
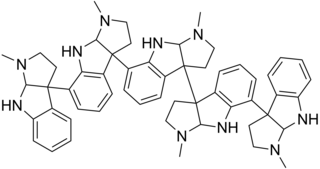
Psychotridine is an alkaloid found in some species of the genus Psychotria, namely Psychotria colorata, but also Psychotria forsteriana, Psychotria lyciiflora, Psychotria oleoides, and Psychotria beccarioides. Psychotridine has analgesic effects and dose-dependently inhibits dizocilpine binding to cortical membranes in vitro, suggesting that it acts as a non-competitive NMDA receptor antagonist.

Huáng bǎi or huáng bò (黄檗) is one of the fifty fundamental herbs of traditional Chinese medicine. Known also as Cortex Phellodendri, it is the bark of one of two species of Phellodendron tree: Phellodendron amurense or Phellodendron chinense.

Eudistomins are β-carboline derivatives, isolated from ascidians, like Ritterella sigillinoides, Lissoclinum fragile, or Pseudodistoma aureum.
Biomimetic synthesis is an area of organic chemical synthesis that is specifically biologically inspired, so-named in 1917 by the English organic chemist and Nobel laureate Sir Robert Robinson. The term encompasses both the testing of a "biogenetic hypothesis" through execution of a series of reactions designed to parallel the proposed biosynthesis, as well as programs of study where a synthetic reaction or reactions aimed at a desired synthetic goal are designed to mimic a one or more known enzymic transformations of an established biosynthetic pathway. The earliest generally cited example of a biomimetic synthesis is Robinson's organic synthesis of the alkaloid tropinone.
The nitronickelates are a class of chemical compounds containing a nickel atom complexed by nitro groups, -NO2. Nickel can be in a +2 or +3 oxidation state. There can be five (pentanitronickelates), or six, (hexanitronickelates) nitro groups per nickel atom. The can be considered the double nitrites of nickel nitrite.


















