A nanowire is a nanostructure in the form of a wire with the diameter of the order of a nanometre (10−9 m). More generally, nanowires can be defined as structures that have a thickness or diameter constrained to tens of nanometers or less and an unconstrained length. At these scales, quantum mechanical effects are important—which coined the term "quantum wires".

In molecular physics and chemistry, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules.

Adhesion is the tendency of dissimilar particles or surfaces to cling to one another.

Nanomaterials describe, in principle, chemical substances or materials of which a single unit is sized between 1 and 100 nm.
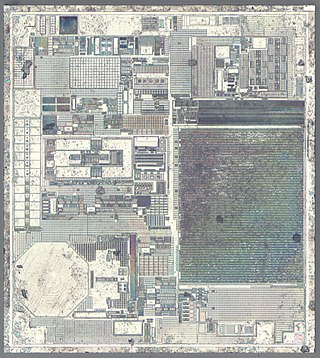
Nanoelectromechanical systems (NEMS) are a class of devices integrating electrical and mechanical functionality on the nanoscale. NEMS form the next logical miniaturization step from so-called microelectromechanical systems, or MEMS devices. NEMS typically integrate transistor-like nanoelectronics with mechanical actuators, pumps, or motors, and may thereby form physical, biological, and chemical sensors. The name derives from typical device dimensions in the nanometer range, leading to low mass, high mechanical resonance frequencies, potentially large quantum mechanical effects such as zero point motion, and a high surface-to-volume ratio useful for surface-based sensing mechanisms. Applications include accelerometers and sensors to detect chemical substances in the air.

Galling is a form of wear caused by adhesion between sliding surfaces. When a material galls, some of it is pulled with the contacting surface, especially if there is a large amount of force compressing the surfaces together. Galling is caused by a combination of friction and adhesion between the surfaces, followed by slipping and tearing of crystal structure beneath the surface. This will generally leave some material stuck or even friction welded to the adjacent surface, whereas the galled material may appear gouged with balled-up or torn lumps of material stuck to its surface.
Fretting refers to wear and sometimes corrosion damage of loaded surfaces in contact while they encounter small oscillatory movements tangential to the surface. Fretting is caused by adhesion of contact surface asperities, which are subsequently broken again by the small movement. This breaking causes wear debris to be formed.

Focused ion beam, also known as FIB, is a technique used particularly in the semiconductor industry, materials science and increasingly in the biological field for site-specific analysis, deposition, and ablation of materials. A FIB setup is a scientific instrument that resembles a scanning electron microscope (SEM). However, while the SEM uses a focused beam of electrons to image the sample in the chamber, a FIB setup uses a focused beam of ions instead. FIB can also be incorporated in a system with both electron and ion beam columns, allowing the same feature to be investigated using either of the beams. FIB should not be confused with using a beam of focused ions for direct write lithography. These are generally quite different systems where the material is modified by other mechanisms.
A break junction is an electronic device which consists of two metal wires separated by a very thin gap, on the order of the inter-atomic spacing. This can be done by physically pulling the wires apart or through chemical etching or electromigration. As the wire breaks, the separation between the electrodes can be indirectly controlled by monitoring the electrical resistance of the junction.

Nanochemistry is an emerging sub-discipline of the chemical and material sciences that deals with the development of new methods for creating nanoscale materials. The term "nanochemistry" was first used by Ozin in 1992 as 'the uses of chemical synthesis to reproducibly afford nanomaterials from the atom "up", contrary to the nanoengineering and nanophysics approach that operates from the bulk "down"'. Nanochemistry focuses on solid-state chemistry that emphasizes synthesis of building blocks that are dependent on size, surface, shape, and defect properties, rather than the actual production of matter. Atomic and molecular properties mainly deal with the degrees of freedom of atoms in the periodic table. However, nanochemistry introduced other degrees of freedom that controls material's behaviors by transformation into solutions. Nanoscale objects exhibit novel material properties, largely as a consequence of their finite small size. Several chemical modifications on nanometer-scaled structures approve size dependent effects.

Thermal spraying techniques are coating processes in which melted materials are sprayed onto a surface. The "feedstock" is heated by electrical or chemical means.
Vacuum cementing or vacuum welding is the natural process of solidifying small objects in a hard vacuum. The most notable example is dust on the surface of the Moon.

Glass-to-metal seals are a type of mechanical seal which joins glass and metal surfaces. They are very important elements in the construction of vacuum tubes, electric discharge tubes, incandescent light bulbs, glass-encapsulated semiconductor diodes, reed switches, glass windows in metal cases, and metal or ceramic packages of electronic components.
Dry lubricants or solid lubricants are materials that, despite being in the solid phase, are able to reduce friction between two surfaces sliding against each other without the need for a liquid oil medium.

Dispersive adhesion, also called adsorptive adhesion, is a mechanism for adhesion which attributes attractive forces between two materials to intermolecular interactions between molecules of each material. This mechanism is widely viewed as the most important of the five mechanisms of adhesion due to its presence in every type of adhesive system and its relative strength.

The vapor–liquid–solid method (VLS) is a mechanism for the growth of one-dimensional structures, such as nanowires, from chemical vapor deposition. The growth of a crystal through direct adsorption of a gas phase on to a solid surface is generally very slow. The VLS mechanism circumvents this by introducing a catalytic liquid alloy phase which can rapidly adsorb a vapor to supersaturation levels, and from which crystal growth can subsequently occur from nucleated seeds at the liquid–solid interface. The physical characteristics of nanowires grown in this manner depend, in a controllable way, upon the size and physical properties of the liquid alloy.
Thermocompression bonding describes a wafer bonding technique and is also referred to as diffusion bonding, pressure joining, thermocompression welding or solid-state welding. Two metals, e.g. gold-gold (Au), are brought into atomic contact applying force and heat simultaneously. The diffusion requires atomic contact between the surfaces due to the atomic motion. The atoms migrate from one crystal lattice to the other one based on crystal lattice vibration. This atomic interaction sticks the interface together. The diffusion process is described by the following three processes:
Direct bonding, or fusion bonding, is a wafer bonding process without any additional intermediate layers. It is based on chemical bonds between two surfaces of any material possible meeting numerous requirements. These requirements are specified for the wafer surface as sufficiently clean, flat and smooth. Otherwise unbonded areas so called voids, i.e. interface bubbles, can occur.
Band-gap engineering is the process of controlling or altering the band gap of a material. This is typically done to semiconductors by controlling the composition of alloys, constructing layered materials with alternating compositions, or by inducing strain either epitaxially or topologically. A band gap is the range in a solid where no electron state can exist. The band gap of insulators is much larger than in semiconductors. Conductors or metals have a much smaller or nonexistent band gap than semiconductors since the valence and conduction bands overlap. Controlling the band gap allows for the creation of desirable electrical properties.

Diffusion bonding or diffusion welding is a solid-state welding technique used in metalworking, capable of joining similar and dissimilar metals. It operates on the principle of solid-state diffusion, wherein the atoms of two solid, metallic surfaces intersperse themselves over time. This is typically accomplished at an elevated temperature, approximately 50-75% of the absolute melting temperature of the materials. A weak bond can also be achieved at room temperature. Diffusion bonding is usually implemented by applying high pressure, in conjunction with necessarily high temperature, to the materials to be welded; the technique is most commonly used to weld "sandwiches" of alternating layers of thin metal foil, and metal wires or filaments. Currently, the diffusion bonding method is widely used in the joining of high-strength and refractory metals within the aerospace and nuclear industries.












