
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934%. It is more than twice as abundant as water vapor, 23 times as abundant as carbon dioxide, and more than 500 times as abundant as neon. Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust.
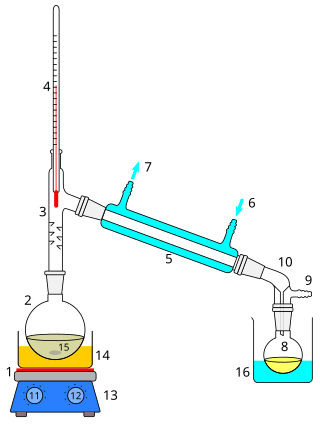
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used.

An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. Though inert gases have a variety of applications, they are generally used to prevent unwanted chemical reactions with the oxygen (oxidation) and moisture (hydrolysis) in the air from degrading a sample. Generally, all noble gases except oganesson, nitrogen, and carbon dioxide are considered inert gases. The term inert gas is context-dependent because several of the noble gases, including nitrogen and carbon dioxide, can be made to react under certain conditions.

Liquid nitrogen (LN2) is nitrogen in a liquid state at low temperature. Liquid nitrogen has a boiling point of about −196 °C (−321 °F; 77 K). It is produced industrially by fractional distillation of liquid air. It is a colorless, mobile liquid whose viscosity is about one-tenth that of acetone (i.e. roughly one-thirtieth that of water at room temperature). Liquid nitrogen is widely used as a coolant.
Cryogenic fuels are fuels that require storage at extremely low temperatures in order to maintain them in a liquid state. These fuels are used in machinery that operates in space where ordinary fuel cannot be used, due to the very low temperatures often encountered in space, and the absence of an environment that supports combustion. Cryogenic fuels most often constitute liquefied gases such as liquid hydrogen.
Liquid air is air that has been cooled to very low temperatures, so that it has condensed into a pale blue mobile liquid. It is stored in specialized containers, such as vacuum flasks, to insulate it from room temperature. Liquid air can absorb heat rapidly and revert to its gaseous state. It is often used for condensing other substances into liquid and/or solidifying them, and as an industrial source of nitrogen, oxygen, argon, and other inert gases through a process called air separation.
An oxygen concentrator is a device that concentrates the oxygen from a gas supply by selectively removing nitrogen to supply an oxygen-enriched product gas stream. They are used industrially, to provide supplemental oxygen at high altitudes, and as medical devices for oxygen therapy.

Pressure swing adsorption (PSA) is a technique used to separate some gas species from a mixture of gases under pressure according to the species' molecular characteristics and affinity for an adsorbent material. It operates at near-ambient temperature and significantly differs from the cryogenic distillation commonly used to separate gases. Selective adsorbent materials are used as trapping material, preferentially adsorbing the target gas species at high pressure. The process then swings to low pressure to desorb the adsorbed gas.

Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). The liquefaction of gases is a complicated process that uses various compressions and expansions to achieve high pressures and very low temperatures, using, for example, turboexpanders.

Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also available in gas cylinders. The industry producing these gases is also known as industrial gas, which is seen as also encompassing the supply of equipment and technology to produce and use the gases. Their production is a part of the wider chemical Industry.

A turboexpander, also referred to as a turbo-expander or an expansion turbine, is a centrifugal or axial-flow turbine, through which a high-pressure gas is expanded to produce work that is often used to drive a compressor or generator.
Huntsman Chemical Company of Australia Pty Ltd (HCCA) operated a complex chemical manufacturing plant in Somerville Rd Brooklyn in Melbourne. The site is 35 hectares in size and is located in the City of Brimbank. HCCA was partially owned by the Huntsman Corporation.
An oil production plant is a facility which processes production fluids from oil wells in order to separate out key components and prepare them for export. Typical oil well production fluids are a mixture of oil, gas and produced water. An oil production plant is distinct from an oil depot, which does not have processing facilities.

Nitrogen generators and stations are stationary or mobile air-to-nitrogen production complexes.
Oxygen plants are industrial systems designed to generate oxygen. They typically use air as a feedstock and separate it from other components of air using pressure swing adsorption or membrane separation techniques. Such plants are distinct from cryogenic separation plants which separate and capture all the components of air.
A liquid nitrogen engine is powered by liquid nitrogen, which is stored in a tank. Traditional nitrogen engine designs work by heating the liquid nitrogen in a heat exchanger, extracting heat from the ambient air and using the resulting pressurized gas to operate a piston or rotary motor. Vehicles propelled by liquid nitrogen have been demonstrated, but are not used commercially. One such vehicle, Liquid Air, was demonstrated in 1902.
An air separation plant separates atmospheric air into its primary components, typically nitrogen and oxygen, and sometimes also argon and other rare inert gases.

Matheson Tri-Gas, Inc. produces industrial, medical, and specialty gases, and associated gas handling equipment, in North America. MATHESON offers semiconductor, medical, welding, atmospheric gases, rare gases delivered via pipelines, onsite generators, bulk tanks, and in gas cylinders to customers using gases in their labs, semiconductor fabs, hospitals, chemical plants, manufacturing and many other processes. Furthermore, MATHESON also designs and manufactures gas purification systems, generators, delivery systems, filters, gas purifiers, detection equipment, control valves, and management accessories; and gas cylinder enclosures, source manifolds, and panels, as well as helium recovery solutions. In addition, the company provides support, engineering, and systems management services to analytical laboratories and semiconductor manufacturers worldwide.
Liquid Nitrogen Wash is a process mainly used for the production of ammonia synthesis gas within fertilizer production plants. It is usually the last purification step in the ammonia production process sequence upstream of the actual ammonia production.













