
A protease is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in numerous biological pathways, including digestion of ingested proteins, protein catabolism, and cell signaling.
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily.
In biology and biochemistry, protease inhibitors, or antiproteases, are molecules that inhibit the function of proteases. Many naturally occurring protease inhibitors are proteins.

Serine proteases are enzymes that cleave peptide bonds in proteins. Serine serves as the nucleophilic amino acid at the (enzyme's) active site. They are found ubiquitously in both eukaryotes and prokaryotes. Serine proteases fall into two broad categories based on their structure: chymotrypsin-like (trypsin-like) or subtilisin-like.

A metalloproteinase, or metalloprotease, is any protease enzyme whose catalytic mechanism involves a metal. An example is ADAM12 which plays a significant role in the fusion of muscle cells during embryo development, in a process known as myogenesis.

Papain, also known as papaya proteinase I, is a cysteine protease enzyme present in papaya and mountain papaya. It is the namesake member of the papain-like protease family.
Ophanin is a toxin found in the venom of the King Cobra, which lives throughout South East Asia. This toxin belongs to the cysteine-rich secretory protein (CRISP) family. Ophanin weakly blocks the contraction of smooth muscles elicited by high potassium-induced depolarization, suggesting that it inhibits voltage-dependent calcium channels.
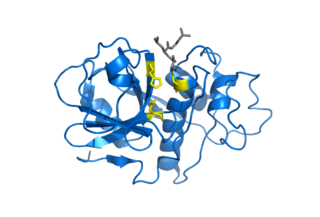
Cysteine proteases, also known as thiol proteases, are hydrolase enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad.

Cathepsin S is a protein that in humans is encoded by the CTSS gene. Transcript variants utilizing alternative polyadenylation signals exist for this gene.
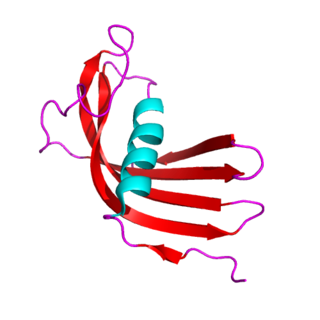
The cystatins are a family of cysteine protease inhibitors which share a sequence homology and a common tertiary structure of an alpha helix lying on top of an anti-parallel beta sheet. The family is subdivided as described below.
In molecular biology, the Signal Peptide Peptidase (SPP) is a type of protein that specifically cleaves parts of other proteins. It is an intramembrane aspartyl protease with the conserved active site motifs 'YD' and 'GxGD' in adjacent transmembrane domains (TMDs). Its sequences is highly conserved in different vertebrate species. SPP cleaves remnant signal peptides left behind in membrane by the action of signal peptidase and also plays key roles in immune surveillance and the maturation of certain viral proteins.

Glia-derived nexin is a protein that in humans is encoded by the SERPINE2 gene.
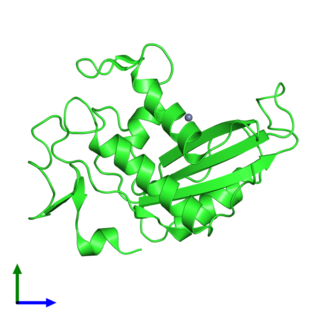
Glioma pathogenesis-related protein 1 is a protein that in humans is encoded by the GLIPR1 gene.
Ablomin is a toxin present in the venom of the Japanese Mamushi snake, which blocks L-type voltage-gated calcium channels.
Triflin is a cysteine-rich secretory protein (CRISP), which is excreted by the venom gland of the Habu snake. Triflin reduces high potassium-induced smooth muscle contraction, suggesting a blocking effect on L-type calcium channels.
Latisemin is a cysteine-rich secretory protein that can be isolated from the venom of the Black-banded sea krait, a sea snake indigenous to the warmer waters of the western Pacific Ocean. It is a toxin that inhibits cyclic nucleotide-gated ion channels and blocks L-type calcium channels, thereby reducing smooth muscle contraction.

Cysteine-rich secretory proteins, often abbreviated as CRISPs, are a group of glycoproteins. They are a subgroup of the CRISP, antigen 5 and Pr-1 (CAP) protein superfamily and also contain a domain related to the ShK toxins. They are substantially implicated in the functioning of the mammalian reproductive system. CRISPs are also found in a variety of snake venoms where they inhibit both smooth muscle contraction and cyclic nucleotide-gated ion channels.

The Kazal domain is an evolutionary conserved protein domain usually indicative of serine protease inhibitors. However, kazal-like domains are also seen in the extracellular part of agrins, which are not known to be protease inhibitors.

Peptidase 1 (mite) (EC 3.4.22.65), also known as endopeptidase 1 (mite), is an enzyme found in various species of mites. This enzyme exhibits cysteine protease activity with broad endopeptidase specificity.

Papain-like proteases are a large protein family of cysteine protease enzymes that share structural and enzymatic properties with the group's namesake member, papain. They are found in all domains of life. In animals, the group is often known as cysteine cathepsins or, in older literature, lysosomal peptidases. In the MEROPS protease enzyme classification system, papain-like proteases form Clan CA. Papain-like proteases share a common catalytic dyad active site featuring a cysteine amino acid residue that acts as a nucleophile.













