
Kringle Domains are autonomous protein domains that fold into large loops stabilized by 3 disulfide linkages. These are important in protein–protein interactions with blood coagulation factors. The name Kringle comes from the Scandinavian pastry that these structures resemble.
Nonsyndromic deafness is hearing loss that is not associated with other signs and symptoms. In contrast, syndromic deafness involves hearing loss that occurs with abnormalities in other parts of the body. Genetic changes are related to the following types of nonsyndromic deafness.
Collectins (collagen-containing C-type lectins) are a part of the innate immune system. They form a family of collagenous Ca2+-dependent defense lectins, which are found in animals. Collectins are soluble pattern recognition receptors (PRRs). Their function is to bind to oligosaccharide structure or lipids that are on the surface of microorganisms. Like other PRRs they bind pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) of oligosaccharide origin. Binding of collectins to microorganisms may trigger elimination of microorganisms by aggregation, complement activation, opsonization, activation of phagocytosis, or inhibition of microbial growth. Other functions of collectins are modulation of inflammatory, allergic responses, adaptive immune system and clearance of apoptotic cells.

Gap junction beta-2 protein (GJB2), also known as connexin 26 (Cx26) — is a protein that in humans is encoded by the GJB2 gene.

Usherin is a protein that in humans is encoded by the USH2A gene.

The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in proteins: these repeats typically fold together to form a single, linear solenoid domain block as a functional unit.

Gap junction beta-6 protein (GJB6), also known as connexin 30 (Cx30) — is a protein that in humans is encoded by the GJB6 gene. Connexin 30 (Cx30) is one of several gap junction proteins expressed in the inner ear. Mutations in gap junction genes have been found to lead to both syndromic and nonsyndromic deafness. Mutations in this gene are associated with Clouston syndrome.
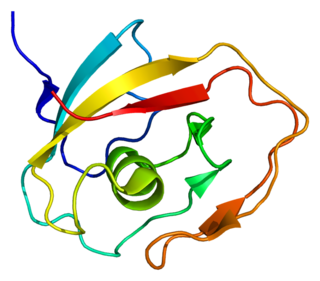
Cochlin is a protein that in humans is encoded by the COCH gene. It is an extracellular matrix (ECM) protein highly abundant in the cochlea and vestibule of the inner ear, constituting the major non-collagen component of the ECM of the inner ear. The protein is highly conserved in human, mouse, and chicken, showing 94% and 79% amino acid identity of human to mouse and chicken sequences, respectively.

Potassium voltage-gated channel subfamily KQT member 4 also known as voltage-gated potassium channel subunit Kv7.4 is a protein that in humans is encoded by the KCNQ4 gene.

Non-syndromic hearing impairment protein 5 is a protein that in humans is encoded by the DFNA5 gene.

Alpha-tectorin is a protein that in humans is encoded by the TECTA gene.

Eyes absent homolog 4 is a protein that in humans is encoded by the EYA4 gene.

Myosin-Ia is a protein that in humans is encoded by the MYO1A gene.

Transmembrane channel-like protein 1 is a protein that in humans is encoded by the TMC1 gene. TMC1 contains six transmembrane domains with both the C and N termini on the endoplasmic side of the membrane, as well as a large loop between domains 4 and 5. This topology is similar to that of transient receptor potential channels (TRPs), a family of proteins involved in the perception of senses such as temperature, taste, pressure, and vision. TMC1 has been located in the post-natal mouse cochlea, and knockouts for TMC1 and TMC2 result in both auditory and vestibular deficits indicating TMC1 is a molecular part of auditory transduction.

POU domain, class 4, transcription factor 3 is a protein that in humans is encoded by the POU4F3 gene.

WAP, kazal, immunoglobulin, kunitz and NTR domain-containing protein 1 is a protein that is encoded by the WFIKKN1 gene. when found in humans.
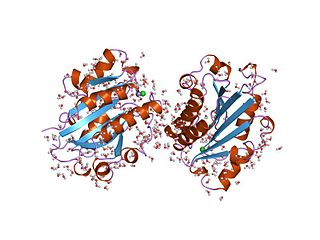
The von Willebrand factor type A (vWA) domain is a protein domain named after its occurrence in von Willebrand factor (vWF), a large multimeric glycoprotein found in blood plasma. Mutant forms of vWF are involved in the aetiology of bleeding disorders. This type A domain is the prototype for a protein superfamily.

Espin, also known as autosomal recessive deafness type 36 protein or ectoplasmic specialization protein, is a protein that in humans is encoded by the ESPN gene. Espin is a microfilament binding protein.
Limulus clotting factor overbar C is an enzyme. This enzyme catalyses the following chemical reaction

WAP, follistatin/kazal, immunoglobulin, kunitz and netrin domain containing 2 is a protein that in humans is encoded by the WFIKKN2 gene.















