Flea, the common name for the order Siphonaptera, includes 2,500 species of small flightless insects that survive as external parasites of mammals and birds. Fleas live by consuming blood, or hematophagy, from their hosts. Adult fleas grow to about 3 millimetres long, are usually brown, and have bodies that are "flattened" sideways or narrow, enabling them to move through their host's fur or feathers. They lack wings, but have strong claws preventing them from being dislodged, mouthparts adapted for piercing skin and sucking blood, and hind legs extremely well adapted for jumping. They are able to leap a distance of some 50 times their body length, a feat second only to jumps made by another group of insects, the superfamily of froghoppers. Flea larvae are worm-like with no limbs; they have chewing mouthparts and feed on organic debris left on their host's skin.

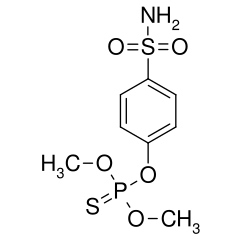
Chlorfenvinphos is the common name of an organophosphorus compound that was widely used as an insecticide and an acaricide. The molecule itself can be described as an enol ester derived from dichloroacetophenone and diethylphosphonic acid. Chlorfenvinphos has been included in many products since its first use in 1963. However, because of its toxic effect as a cholinesterase inhibitor it has been banned in several countries, including the United States and the European Union. Its use in the United States was cancelled in 1991.

Fipronil is a broad-spectrum insecticide that belongs to the phenylpyrazole chemical family. Fipronil disrupts the insect central nervous system by blocking GABA-gated chloride channels and glutamate-gated chloride (GluCl) channels. This causes hyperexcitation of contaminated insects' nerves and muscles. Fipronil's specificity towards insects is believed to be due to its greater affinity to the GABAA receptors of insects, than to those of mammals, and to its action on GluCl channels, which do not exist in mammals. As of 2017 there did not appear to be significant resistance among fleas to fipronil.

Lufenuron is the active ingredient in the veterinary flea control medication program, and one of the two active ingredients in the flea, heartworm, ringworm and anthelmintic medicine milbemycin oxime/lufenuron (Sentinel).

The cat flea is an extremely common parasitic insect whose principal host is the domestic cat, although a high proportion of the fleas found on dogs also belong to this species. This is despite the widespread existence of a separate and well-established "dog" flea, Ctenocephalides canis. Cat fleas can be found globally.

Pyriproxyfen is a pesticide which is found to be effective against a variety of insects. It was introduced to the US in 1996, to protect cotton crops against whitefly. It has also been found useful for protecting other crops. It is also used as a prevention for flea control on household pets, for killing indoor and outdoor ants and roaches. Methods of application include aerosols, bait, carpet powders, foggers, shampoos and pet collars.
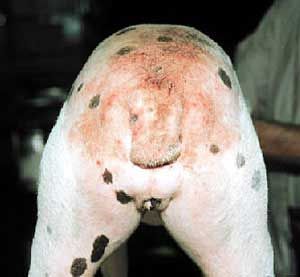
Flea allergy dermatitis (FAD) is an eczematous itchy skin disease of dogs and cats. For both of these domestic species, flea allergy dermatitis is the most common cause of skin disease. Affected animals develop allergic reactions to chemicals in flea saliva. Symptoms of this reaction include erythema (redness), papules (bumps), pustules, and crusts (scabs). If severe, hair loss will occur in the affected area. Dogs with flea allergy dermatitis often show hair loss and eczematous skin rash on the lower back, upper tail, neck, and down the back of the legs. Cats with flea allergy dermatitis may develop a variety of skin problems, including feline eosinophilic granuloma, miliary dermatitis, or self-inflicted alopecia from excessive grooming.

Benzoylureas are chemical derivatives of N-benzoyl-N′-phenylurea (benzoylurea). They are best known for their use as insecticides. They act as insect growth regulators by inhibiting synthesis of chitin in the insect's body.

Spinosad is an insecticide based on chemical compounds found in the bacterial species Saccharopolyspora spinosa. The genus Saccharopolyspora was discovered in 1985 in isolates from crushed sugarcane. The bacteria produce yellowish-pink aerial hyphae, with bead-like chains of spores enclosed in a characteristic hairy sheath. This genus is defined as aerobic, Gram-positive, nonacid-fast actinomycetes with fragmenting substrate mycelium. S. spinosa was isolated from soil collected inside a nonoperational sugar mill rum still in the Virgin Islands. Spinosad is a mixture of chemical compounds in the spinosyn family that has a generalized structure consisting of a unique tetracyclic ring system attached to an amino sugar (D-forosamine) and a neutral sugar (tri-Ο-methyl-L-rhamnose). Spinosad is relatively nonpolar and not easily dissolved in water.

Nitenpyram is a chemical frequently used as an insecticide in agriculture and veterinary medicine. The compound is an insect neurotoxin belonging to the class of neonicotinoids which works by blocking neural signaling of the central nervous system. It does so by binding irreversibly to the nicotinic acetylcholine receptor (nACHr) causing a stop of the flow of ions in the postsynaptic membrane of neurons leading to paralysis and death. Nitenpyram is highly selective towards the variation of the nACHr which insects possess, and has seen extensive use in targeted, insecticide applications.

The health of domestic cats is a well studied area in veterinary medicine.
ATCvet code QP53Ectoparasiticides, including insecticides and repellents is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System for veterinary medicinal products, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products for veterinary use. Subgroup QP53 is part of the anatomical group QP Antiparasitic products, insecticides and repellents.
The milbemycins are a group of macrolides chemically related to the avermectins and were first isolated in 1972 from Streptomyces hygroscopicus. They are used in veterinary medicine as antiparasitic agents against worms, ticks and fleas.

Pyriprole is for veterinary use on dogs against external parasites such as fleas and ticks.
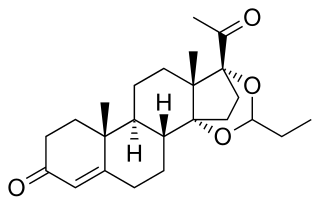
Proligestone, sold under the brand names Covinan and Delvosteron, is a progestin medication which is used in veterinary medicine.

Metaflumizone is a semicarbazone insecticide indicated for the veterinary treatment of fleas and ticks, marketed under the brand name ProMeris. A discontinued variant of ProMeris, called ProMeris Duo or Promeris for Dogs, was indicated for canine use and was a formulated blend of metaflumizone and amitraz. The metaflumizone-only formulation is waterproof and typically remain effective for 30–45 days in a cutaneous application at the base of the neck.

Dinotefuran is an insecticide of the neonicotinoid class developed by Mitsui Chemicals for control of insect pests such as aphids, whiteflies, thrips, leafhoppers, leafminers, sawflies, mole cricket, white grubs, lacebugs, billbugs, beetles, mealybugs, and cockroaches on leafy vegetables, in residential and commercial buildings, and for professional turf management. Its mechanism of action involves disruption of the insect's nervous system by inhibiting nicotinic acetylcholine receptors. In order to avoid harming beneficial insects such as bees, it should not be applied during bloom.

Flumethrin is a pyrethroid insecticide. It is used externally in veterinary medicine against parasitic insects and ticks on cattle, sheep, goats, horses, and dogs, and the treatment of parasitic mites in honeybee colonies.
Flea treatments are procedures used to treat flea infestations in human or animal populations. They may treat both the itching caused by bites and may remove or kill the fleas themselves.
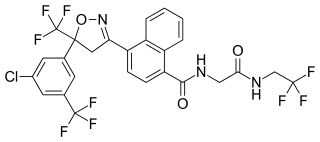
Afoxolaner (INN) is an insecticide and acaricide that belongs to the isoxazoline chemical compound group.