
The danger model of the immune system proposes that it differentiates between components that are capable of causing damage, rather than distinguishing between self and non-self.

The danger model of the immune system proposes that it differentiates between components that are capable of causing damage, rather than distinguishing between self and non-self.
The first major immunologic model was the Self/Non-self Model proposed by Macfarlane Burnet and Frank Fenner in 1949 with later refinement by Burnet. [1] [2] It theorizes that the immune system distinguishes between self, which is tolerated, and non-self, which is attacked and destroyed. According to this theory, the chief cell of the immune system is the B cell, activated by recognizing non-self structures. Later research showed that B cell activation is reliant on CD4+ T helper cells and a co-stimulatory signal from an antigen-presenting cell (APC). Because APCs are not antigen-specific, capable of processing self structures, Charles Janeway proposed the Infectious Non-self Model in 1989. [3] Janeway's theory involved APCs being activated by pattern recognition receptors (PRRs) that recognize evolutionarily conserved pathogen-associated molecular patterns (PAMPs) as infectious non-self, whereas PRRs are not activated by non-infectious self. However, neither of these models are sufficient to explain non-cytopathic viral infections, graft rejection, or anti-tumor immunity. [4]
In 1994, Polly Matzinger formulated the danger model, theorizing that the immune system identifies threats to initiate an immune response based on the presence of pathogens and/or alarm signals from cells under stress. [5] [6] When injured or stressed, tissues typically undergo non-silent types of cell death, such as necrosis or pyroptosis, releasing danger signals like DNA, RNA, heat shock proteins (Hsps), hyaluronic acid, serum amyloid A protein, ATP, uric acid, and cytokines like interferon-α, interleukin-1β, and CD40L for detection by dendritic cells. [4] [6] [7] In comparison, neoplastic tumors do not induce significant immune responses because controlled apoptosis degrades most danger signals, preventing the detection and destruction of malignant cells. [8]
Matzinger's work emphasizes that bodily tissues are the drivers of immunity, providing alarm signals on the location and extent of damage to minimize collateral damage. [9] [10] The adaptive immune system relies on the innate immune system using its antigen-presenting cells to activate B and T lymphocytes for specific antibodies, exemplified by low dendritic cell counts resulting in common variable immunodeficiency (CVID). [11] For example, gut cells secrete transforming growth factor beta (TGF-β) during bacterial invasions to stimulate B cell production of Immunoglobulin A (IgA). [12] Similarly, 30-40% of the liver's T cells are Type I Natural Killer T (NTK) cells, providing Interleukin 4 (IL-4) for an organ-specific response of driving naïve CD4+ T cells to become Type 2 Helper T cells, as opposed to Type 1. [13] [14]
Whereas the danger model proposes non-silent cell death releasing intracellular contents and/or expressing unique signalling proteins to stimulate an immune response, the damage-associated molecular pattern (DAMP) model theorizes that the immune system responds to exposed hydrophobic regions of biological molecules. In 2004, Seung-Yong Seong and Matzinger argued that as cellular damage causes denaturing and protein misfolding, exposed hydrophobic regions aggregate into clumps for improved binding to immune receptors. [15]
Pattern Recognition Receptors (PRRs) are a family of surface receptors on antigen-presenting cells that includes toll-like receptors (TLRs), nucleotide oligomerization domain (NOD)-like receptors, [16] retinoic acid inducible gene-I (RIG-I)-like receptors [17] and C-type lectin-like receptors (CLRs). [18] They recognize alarmins, a category that includes both DAMPs and PAMPs, to process their antigenic regions for presentation to T helper cells. [6]

In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.

The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinters, distinguishing them from the organism's own healthy tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use molecules and cells to perform their functions.

Immunology is a branch of biology and medicine that covers the study of immune systems in all organisms.
An immune response is a physiological reaction which occurs within an organism in the context of inflammation for the purpose of defending against exogenous factors. These include a wide variety of different toxins, viruses, intra- and extracellular bacteria, protozoa, helminths, and fungi which could cause serious problems to the health of the host organism if not cleared from the body.

The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases.

Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-spanning receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13. Humans lack genes for TLR11, TLR12 and TLR13 and mice lack a functional gene for TLR10. The receptors TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in intracellular vesicles.

Polly Celine Eveline Matzinger is a French-born immunologist who proposed the danger model theory of how the immune system works.
Pathogen-associated molecular patterns (PAMPs) are small molecular motifs conserved within a class of microbes, but not present in the host. They are recognized by toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) in both plants and animals. This allows the innate immune system to recognize pathogens and thus, protect the host from infection.
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed mainly by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils, as well as by epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.

The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.
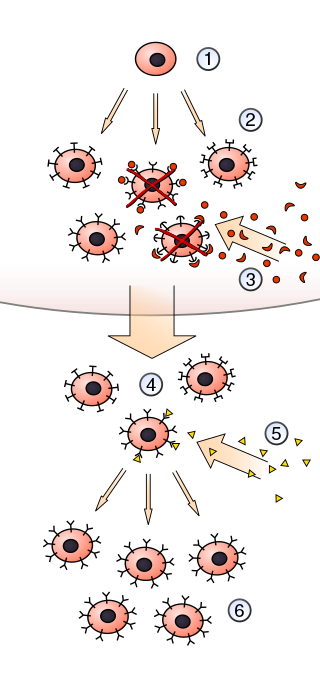
In immunology, clonal selection theory explains the functions of cells of the immune system (lymphocytes) in response to specific antigens invading the body. The concept was introduced by Australian doctor Frank Macfarlane Burnet in 1957, in an attempt to explain the great diversity of antibodies formed during initiation of the immune response. The theory has become the widely accepted model for how the human immune system responds to infection and how certain types of B and T lymphocytes are selected for destruction of specific antigens.
Gut-associated lymphoid tissue (GALT) is a component of the mucosa-associated lymphoid tissue (MALT) which works in the immune system to protect the body from invasion in the gut.

The innate immune system or nonspecific immune system is one of the two main immunity strategies in vertebrates. The innate immune system is an alternate defense strategy and is the dominant immune system response found in plants, fungi, prokaryotes, and invertebrates.
Molecular mimicry is the theoretical possibility that sequence similarities between foreign and self-peptides are enough to result in the cross-activation of autoreactive T or B cells by pathogen-derived peptides. Despite the prevalence of several peptide sequences which can be both foreign and self in nature, just a few crucial residues can activate a single antibody or TCR. This highlights the importance of structural homology in the theory of molecular mimicry. Upon activation, these "peptide mimic" specific T or B cells can cross-react with self-epitopes, thus leading to tissue pathology (autoimmunity). Molecular mimicry is one of several ways in which autoimmunity can be evoked. A molecular mimicking event is more than an epiphenomenon despite its low probability, and these events have serious implications in the onset of many human autoimmune disorders.

Interleukin 19 (IL-19) is an immunosuppressive protein that belongs to the IL-10 cytokine subfamily.
In immunology, an adjuvant is a substance that increases or modulates the immune response to a vaccine. The word "adjuvant" comes from the Latin word adiuvare, meaning to help or aid. "An immunologic adjuvant is defined as any substance that acts to accelerate, prolong, or enhance antigen-specific immune responses when used in combination with specific vaccine antigens."
In immunology, peripheral tolerance is the second branch of immunological tolerance, after central tolerance. It takes place in the immune periphery. Its main purpose is to ensure that self-reactive T and B cells which escaped central tolerance do not cause autoimmune disease. Peripheral tolerance can also serve a purpose in preventing an immune response to harmless food antigens and allergens.

RIG-I is a cytosolic pattern recognition receptor (PRR) that can mediate induction of a type-I interferon (IFN1) response. RIG-I is an essential molecule in the innate immune system for recognizing cells that have been infected with a virus. These viruses can include West Nile virus, Japanese Encephalitis virus, influenza A, Sendai virus, flavivirus, and coronaviruses.
Damage-associated molecular patterns (DAMPs) are molecules within cells that are a component of the innate immune response released from damaged or dying cells due to trauma or an infection by a pathogen. They are also known as danger signals, and alarmins because they serve as warning signs to alert the organism to any damage or infection to its cells. DAMPs are endogenous danger signals that are discharged to the extracellular space in response to damage to the cell from mechanical trauma or a pathogen. Once a DAMP is released from the cell, it promotes a noninfectious inflammatory response by binding to a pattern recognition receptor (PRR). Inflammation is a key aspect of the innate immune response; it is used to help mitigate future damage to the organism by removing harmful invaders from the affected area and start the healing process. As an example, the cytokine IL-1α is a DAMP that originates within the nucleus of the cell which, once released to the extracellular space, binds to the PRR IL-1R, which in turn initiates an inflammatory response to the trauma or pathogen that initiated the release of IL-1α. In contrast to the noninfectious inflammatory response produced by DAMPs, pathogen-associated molecular patterns (PAMPs) initiate and perpetuate the infectious pathogen-induced inflammatory response. Many DAMPs are nuclear or cytosolic proteins with defined intracellular function that are released outside the cell following tissue injury. This displacement from the intracellular space to the extracellular space moves the DAMPs from a reducing to an oxidizing environment, causing their functional denaturation, resulting in their loss of function. Outside of the aforementioned nuclear and cytosolic DAMPs, there are other DAMPs originated from different sources, such as mitochondria, granules, the extracellular matrix, the endoplasmic reticulum, and the plasma membrane.
Induced-self antigen is a marker of abnormal self, which can be recognized upon infected and transformed cells. Therefore, the recognition of "induced self" is an important strategy for surveillance of infection or tumor transformation - it results in elimination of the affected cells by activated NK cells or other immunological mechanisms. Similarly γδ T cells can recognize induced-self antigens expressed on cells under stress conditions.