Related Research Articles

In immunology,an antigen (Ag) is a molecule,moiety,foreign particulate matter,or an allergen,such as pollen,that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.

The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens,from viruses to parasitic worms,as well as cancer cells and objects such as wood splinters,distinguishing them from the organism's own healthy tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use molecules and cells to perform their functions.
An immune response is a physiological reaction which occurs within an organism in the context of inflammation for the purpose of defending against exogenous factors. These include a wide variety of different toxins,viruses,intra- and extracellular bacteria,protozoa,helminths,and fungi which could cause serious problems to the health of the host organism if not cleared from the body.

Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-spanning receptors usually expressed on sentinel cells such as macrophages and dendritic cells,that recognize structurally conserved molecules derived from microbes. Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa,they are recognized by TLRs,which activate immune cell responses. The TLRs include TLR1,TLR2,TLR3,TLR4,TLR5,TLR6,TLR7,TLR8,TLR9,TLR10,TLR11,TLR12,and TLR13. Humans lack genes for TLR11,TLR12 and TLR13 and mice lack a functional gene for TLR10. The receptors TLR1,TLR2,TLR4,TLR5,TLR6,and TLR10 are located on the cell membrane,whereas TLR3,TLR7,TLR8,and TLR9 are located in intracellular vesicles.

Polly Celine Eveline Matzinger is a French-born immunologist who proposed the danger model theory of how the immune system works.
Opsonins are extracellular proteins that,when bound to substances or cells,induce phagocytes to phagocytose the substances or cells with the opsonins bound. Thus,opsonins act as tags to label things in the body that should be phagocytosed by phagocytes. Different types of things ("targets") can be tagged by opsonins for phagocytosis,including:pathogens,cancer cells,aged cells,dead or dying cells,excess synapses,or protein aggregates. Opsonins help clear pathogens,as well as dead,dying and diseased cells.
Pathogen-associated molecular patterns (PAMPs) are small molecular motifs conserved within a class of microbes,but not present in the host. They are recognized by toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) in both plants and animals. This allows the innate immune system to recognize pathogens and thus,protect the host from infection.
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors,which detect molecules typical for the pathogens. They are proteins expressed mainly by cells of the innate immune system,such as dendritic cells,macrophages,monocytes,neutrophils,as well as by epithelial cells,to identify two classes of molecules:pathogen-associated molecular patterns (PAMPs),which are associated with microbial pathogens,and damage-associated molecular patterns (DAMPs),which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system,particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.
Scavenger receptors are a large and diverse superfamily of cell surface receptors. Its properties were first recorded in 1970 by Drs. Brown and Goldstein,with the defining property being the ability to bind and remove modified low density lipoproteins (LDL). Today scavenger receptors are known to be involved in a wide range of processes,such as:homeostasis,apoptosis,inflammatory diseases and pathogen clearance. Scavenger receptors are mainly found on myeloid cells and other cells that bind to numerous ligands,primarily endogenous and modified host-molecules together with pathogen-associated molecular patterns(PAMPs),and remove them. The Kupffer cells in the liver are particularly rich in scavenger receptors,includes SR-A I,SR-A II,and MARCO.

The innate, or nonspecific,immune system is one of the two main immunity strategies in vertebrates. The innate immune system is an alternate defense strategy and is the dominant immune system response found in plants,fungi,prokaryotes,and invertebrates.

Ellen Heber-Katz is an American immunologist and regeneration biologist who is a professor at Lankenau Institute for Medical Research (LIMR). She discovered that the Murphy Roths Large (MRL) mouse strain can regenerate wounds without scarring,and can fully restore damaged tissues. Her work on regeneration has been extended into National Cancer Institute (NCI)-funded studies of novel aspects of breast cancer causation. Her research interests include immunology,regenerative medicine and cancer.

The nucleotide-binding oligomerization domain-like receptors,or NOD-like receptors (NLRs),are intracellular sensors of pathogen-associated molecular patterns (PAMPs) that enter the cell via phagocytosis or pores,and damage-associated molecular patterns (DAMPs) that are associated with cell stress. They are types of pattern recognition receptors (PRRs),and play key roles in the regulation of innate immune response. NLRs can cooperate with toll-like receptors (TLRs) and regulate inflammatory and apoptotic response.
Damage-associated molecular patterns (DAMPs) are molecules within cells that are a component of the innate immune response released from damaged or dying cells due to trauma or an infection by a pathogen. They are also known as danger signals,and alarmins because they serve as warning signs to alert the organism to any damage or infection to its cells. DAMPs are endogenous danger signals that are discharged to the extracellular space in response to damage to the cell from mechanical trauma or a pathogen. Once a DAMP is released from the cell,it promotes a noninfectious inflammatory response by binding to a pattern recognition receptor. Inflammation is a key aspect of the innate immune response;it is used to help mitigate future damage to the organism by removing harmful invaders from the affected area and start the healing process. As an example,the cytokine IL-1αis a DAMP that originates within the nucleus of the cell which,once released to the extracellular space,binds to the PRR IL-1R,which in turn initiates an inflammatory response to the trauma or pathogen that initiated the release of IL-1α. In contrast to the noninfectious inflammatory response produced by DAMPs,pathogen-associated molecular patterns initiate and perpetuate the infectious pathogen-induced inflammatory response. Many DAMPs are nuclear or cytosolic proteins with defined intracellular function that are released outside the cell following tissue injury. This displacement from the intracellular space to the extracellular space moves the DAMPs from a reducing to an oxidizing environment,causing their functional denaturation,resulting in their loss of function. Outside of the aforementioned nuclear and cytosolic DAMPs,there are other DAMPs originated from different sources,such as mitochondria,granules,the extracellular matrix,the endoplasmic reticulum,and the plasma membrane.

Inflammasomes are cytosolic multiprotein oligomers of the innate immune system responsible for the activation of inflammatory responses. Activation and assembly of the inflammasome promotes proteolytic cleavage,maturation and secretion of pro-inflammatory cytokines interleukin 1β(IL-1β) and interleukin 18 (IL-18),as well as cleavage of gasdermin D. The N-terminal fragment resulting from this cleavage induces a pro-inflammatory form of programmed cell death distinct from apoptosis,referred to as pyroptosis,and is responsible for secretion of the mature cytokines,presumably through the formation of pores in the plasma membrane. Additionally,inflammasomes can be incorporated into larger cell death-inducing complexes called PANoptosomes,which drive another distinct form of pro-inflammatory cell death called PANoptosis.
Apoptotic-cell associated molecular patterns (ACAMPs) are molecular markers present on cells which are going through apoptosis,i.e. programmed cell death. The term was used for the first time by C. D. Gregory in 2000. Recognition of these patterns by the pattern recognition receptors (PRRs) of phagocytes then leads to phagocytosis of the apoptotic cell. These patterns include eat-me signals on the apoptotic cells,loss of don’t-eat-me signals on viable cells and come-get-me signals ) secreted by the apoptotic cells in order to attract phagocytes. Thanks to these markers,apoptotic cells,unlike necrotic cells,do not trigger the unwanted immune response.

The danger model of the immune system proposes that it differentiates between components that are capable of causing damage,rather that distinguishing between self and non-self.
Immunogenic cell death is any type of cell death eliciting an immune response. Both accidental cell death and regulated cell death can result in immune response. Immunogenic cell death contrasts to forms of cell death that do not elicit any response or even mediate immune tolerance.
Immunological memory is the ability of the immune system to quickly and specifically recognize an antigen that the body has previously encountered and initiate a corresponding immune response. Generally,they are secondary,tertiary and other subsequent immune responses to the same antigen. The adaptive immune system and antigen-specific receptor generation are responsible for adaptive immune memory.
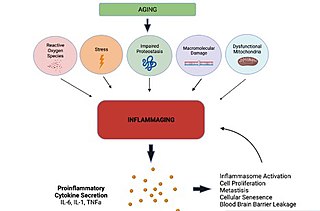
Inflammaging is a chronic,sterile,low-grade inflammation that develops with advanced age,in the absence of overt infection,and may contribute to clinical manifestations of other age-related pathologies. Inflammaging is thought to be caused by a loss of control over systemic inflammation resulting in chronic overstimulation of the innate immune system. Inflammaging is a significant risk factor in mortality and morbidity in aged individuals.
Eui-Cheol Shin is a Korean medical immunologist,academic,and author. He is a professor at the Graduate School of Medical Science and Engineering at the Korea Advanced Institute of Science and Technology (KAIST),and director of The Center for Viral Immunology at the Institute for Basic Science (IBS),a Korean government-funded research institute.
References
- 1 2 "성승용교수, 인체 면역 새이론 '네이처…'誌에 게재". The Dong-a Ilbo . 3 June 2004. Retrieved 26 March 2018.
- 1 2 3 4 5 6 7 Seong, Seung-Yong; Matzinger, Polly (2004). "Opinion: Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses". Nature Reviews Immunology. 4 (6): 469–478. doi:10.1038/nri1372. ISSN 1474-1741. PMID 15173835. S2CID 13336660.
- ↑ Seong, Seung-Yong; Choi, Myung-Sik; Kim, Ik-Sang (2001). "Orientia tsutsugamushi infection:overview and immune responses". Microbes and Infection. 3 (1): 11–21. doi: 10.1016/s1286-4579(00)01352-6 . PMID 11226850.
- 1 2 Seong, Seung-Yong; Cho, Nam-Hyuk; Kwon, Ick Chan; Jeong, Seo Young (1999-07-01). "Protective Immunity of Microsphere-Based Mucosal Vaccines against Lethal Intranasal Challenge with Streptococcus pneumoniae". Infection and Immunity. 67 (7): 3587–3592. doi:10.1128/iai.67.7.3587-3592.1999. ISSN 0019-9567. PMC 116548 . PMID 10377143.
- 1 2 3 4 5 서울대학교의과대학. "Seoul National University College of Medicine". en.medicine.snu.ac.kr. Retrieved 2017-11-30.
- 1 2 "Editorial Board Members". World Journal of Immunology– via Baishideng Publishing Group Inc.
- 1 2 Cho, Nam-Hyuk; Seong, Seung-Yong (2009-09-01). "Apolipoproteins inhibit the innate immunity activated by necrotic cells or bacterial endotoxin". Immunology. 128 (1pt2): e479–e486. doi:10.1111/j.1365-2567.2008.03002.x. ISSN 1365-2567. PMC 2753934 . PMID 19191905.
- ↑ Tang, Daolin; Kang, Rui; Coyne, Carolyn B.; Zeh, Herbert J.; Lotze, Michael T. (2012-09-01). "PAMPs and DAMPs: signal 0s that spur autophagy and immunity". Immunological Reviews. 249 (1): 158–175. doi:10.1111/j.1600-065x.2012.01146.x. ISSN 1600-065X. PMC 3662247 . PMID 22889221.