Related Research Articles
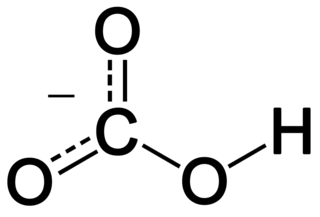
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula HCO−
3.
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula CO2−3. The word carbonate may also refer to a carbonate ester, an organic compound containing the carbonate groupO=C(−O−)2.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Brine is a high-concentration solution of salt in water. In diverse contexts, brine may refer to the salt solutions ranging from about 3.5% up to about 26%. Brine forms naturally due to evaporation of ground saline water but it is also generated in the mining of sodium chloride. Brine is used for food processing and cooking, for de-icing of roads and other structures, and in a number of technological processes. It is also a by-product of many industrial processes, such as desalination, so it requires wastewater treatment for proper disposal or further utilization.

Sodium chloride, commonly known as table salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is deicing of roadways in sub-freezing weather.

In chemistry, there are three definitions in common use of the word "base": Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the Chlor-alkali process.

Hard water is water that has high mineral content. Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates.

Magnesium chloride is an inorganic compound with the formula MgCl2. It forms hydrates MgCl2·nH2O, where n can range from 1 to 12. These salts are colorless or white solids that are highly soluble in water. These compounds and their solutions, both of which occur in nature, have a variety of practical uses. Anhydrous magnesium chloride is the principal precursor to magnesium metal, which is produced on a large scale. Hydrated magnesium chloride is the form most readily available.

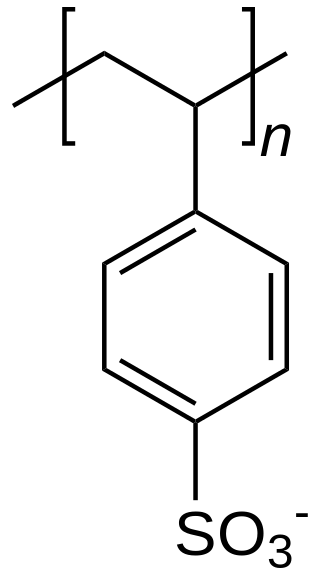
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange. It is an insoluble matrix normally in the form of small microbeads, usually white or yellowish, fabricated from an organic polymer substrate. The beads are typically porous, providing a large surface area on and inside them where the trapping of ions occurs along with the accompanying release of other ions, and thus the process is called ion exchange. There are multiple types of ion-exchange resin. Most commercial resins are made of polystyrene sulfonate, followed up by polyacrylate.

Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings. Water softening is usually achieved using lime softening or ion-exchange resins but is increasingly being accomplished using nanofiltration or reverse osmosis membranes.

Alkalinity (from Arabic: القلوي, romanized: al-qaly, lit. 'ashes of the saltwort') is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. It is measured by titrating the solution with an acid such as HCl until its pH changes abruptly, or it reaches a known endpoint where that happens. Alkalinity is expressed in units of concentration, such as meq/L (milliequivalents per liter), μeq/kg (microequivalents per kilogram), or mg/L CaCO3 (milligrams per liter of calcium carbonate). Each of these measurements corresponds to an amount of acid added as a titrant.

Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances.
In chemistry, a counterion is the ion that accompanies an ionic species in order to maintain electric neutrality. In table salt the sodium ion is the counterion for the chloride ion and vice versa.
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in Nomenclature of Inorganic Chemistry. Ideally, every inorganic compound should have a name from which an unambiguous formula can be determined. There is also an IUPAC nomenclature of organic chemistry.

Electrodialysis (ED) is used to transport salt ions from one solution through ion-exchange membranes to another solution under the influence of an applied electric potential difference. This is done in a configuration called an electrodialysis cell. The cell consists of a feed (dilute) compartment and a concentrate (brine) compartment formed by an anion exchange membrane and a cation exchange membrane placed between two electrodes. In almost all practical electrodialysis processes, multiple electrodialysis cells are arranged into a configuration called an electrodialysis stack, with alternating anion and cation-exchange membranes forming the multiple electrodialysis cells. Electrodialysis processes are different from distillation techniques and other membrane based processes in that dissolved species are moved away from the feed stream, whereas other processes move away the water from the remaining substances. Because the quantity of dissolved species in the feed stream is far less than that of the fluid, electrodialysis offers the practical advantage of much higher feed recovery in many applications.

Boiler water is liquid water within a boiler, or in associated piping, pumps and other equipment, that is intended for evaporation into steam. The term may also be applied to raw water intended for use in boilers, treated boiler feedwater, steam condensate being returned to a boiler, or boiler blowdown being removed from a boiler.

Alkali, or Alkaline, soils are clay soils with high pH, a poor soil structure and a low infiltration capacity. Often they have a hard calcareous layer at 0.5 to 1 metre depth. Alkali soils owe their unfavorable physico-chemical properties mainly to the dominating presence of sodium carbonate, which causes the soil to swell and difficult to clarify/settle. They derive their name from the alkali metal group of elements, to which sodium belongs, and which can induce basicity. Sometimes these soils are also referred to as alkaline sodic soils.
Alkaline soils are basic, but not all basic soils are alkaline.
Chlorine gas can be produced by extracting from natural materials, including the electrolysis of a sodium chloride solution (brine) and other ways.

Concrete degradation may have many different causes. Concrete is mostly damaged by the corrosion of reinforcement bars due to the carbonatation of hardened cement paste or chloride attack under wet conditions. Chemical damages are caused by the formation of expansive products produced by various chemical reactions, by aggressive chemical species present in groundwater and seawater, or by microorganisms. Other damaging processes can also involve calcium leaching by water infiltration and different physical phenomena initiating cracks formation and propagation. All these detrimental processes and damaging agents adversely affects the concrete mechanical strength and its durability.
References
- ↑ Owens, Dean L. (1985). Practical Principles of Ion Exchange Water Treatment. Tall Oaks Publishing. ISBN 0-927188-00-7. LCCN 85051869.
- ↑ "Fleck Water Softener". Saturday, 13 March 2021