Polybrominated diphenyl ethers or PBDEs, are a class of organobromine compounds that are used as flame retardants. Like other brominated flame retardants, PBDEs have been used in a wide array of products, including building materials, electronics, furnishings, motor vehicles, airplanes, plastics, polyurethane foams, and textiles. They are structurally akin to polychlorinated diphenyl ethers (PCDEs), polychlorinated biphenyls (PCBs) and other polyhalogenated compounds, consisting of two halogenated aromatic rings. PBDEs are classified according to the average number of bromine atoms in the molecule. The health hazards of these chemicals have attracted increasing scrutiny, and they have been shown to reduce fertility in humans at levels found in households. Because of their toxicity and persistence, the industrial production of some PBDEs is restricted under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POPs).
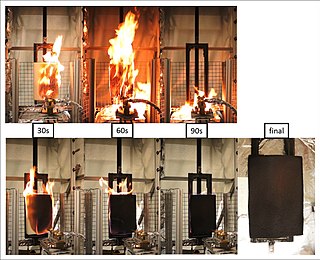
The term flame retardants subsumes a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source and are intended to prevent or slow the further development of ignition by a variety of different physical and chemical methods. They may be added as a copolymer during the polymerisation process, or later added to the polymer at a moulding or extrusion process or applied as a topical finish. Mineral flame retardants are typically additive while organohalogen and organophosphorus compounds can be either reactive or additive.
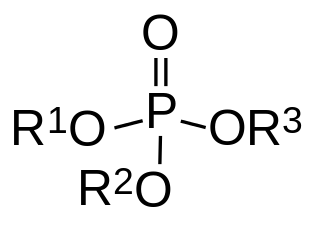
In organic chemistry, organophosphates are a class of organophosphorus compounds with the general structure O=P(OR)3, a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid.
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes.

Persistent organic pollutants (POPs) are organic compounds that are resistant to degradation through chemical, biological, and photolytic processes. They are toxic chemicals that adversely affect human health and the environment around the world. Because they can be transported by wind and water, most POPs generated in one country can and do affect people and wildlife far from where they are used and released.
Brominated flame retardants (BFRs) are organobromine compounds that have an inhibitory effect on combustion chemistry and tend to reduce the flammability of products containing them. The brominated variety of commercialized chemical flame retardants comprise approximately 19.7% of the market. They are effective in plastics and textile applications like electronics, clothes and furniture.
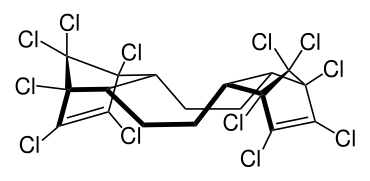
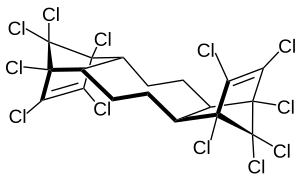
Hexabromocyclododecane is a brominated flame retardant. It consists of twelve carbon, eighteen hydrogen, and six bromine atoms tied to the ring. Its primary application is in extruded (XPS) and expanded (EPS) polystyrene foam that is used as thermal insulation in the building industry. Other uses are upholstered furniture, automobile interior textiles, car cushions and insulation blocks in trucks, packaging material, video cassette recorder housing and electric and electronic equipment. According to UNEP, "HBCD is produced in China, Europe, Japan, and the USA. The known current annual production is approximately 28,000 tonnes per year. The main share of the market volume is used in Europe and China". Due to its persistence, toxicity, and ecotoxicity, the Stockholm Convention on Persistent Organic Pollutants decided in May 2013 to list hexabromocyclododecane in Annex A to the convention with specific exemptions for production and use in expanded polystyrene and extruded polystyrene in buildings. Because HBCD has 16 possible stereo-isomers with different biological activities, the substance poses a difficult problem for manufacture and regulation.
Chlorinated paraffins (CPs) are complex mixtures of polychlorinated n-alkanes. The chlorination degree of CPs can vary between 30 and 70 wt%. CPs are subdivided according to their carbon chain length into short-chain CPs, medium-chain CPs and long-chain CPs. Depending on chain length and chlorine content, CPs are colorless or yellowish liquids or solids.

Decabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). It was commercialised in the 1970s and was initially thought to be safe, but is now recognised as a hazardous and persistent pollutant. It was added to Annex A of the Stockholm Convention on Persistent Organic Pollutants in 2017, which means that treaty members must take measures to eliminate its production and use. The plastics industry started switching to decabromodiphenyl ethane as an alternative in the 1990s, but this is now also coming under regulatory pressure due to concerns over human health.
Pentabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). Because of their toxicity and persistence, their industrial production is to be eliminated under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POP).

Triphenyl phosphate (TPhP) is the chemical compound with the formula OP(OC6H5)3. It is the simplest aromatic organophosphate. This colourless solid is the ester (triester) of phosphoric acid and phenol. It is used as a plasticizer and a fire retardant in a wide variety of settings and products.

Bisphenol S (BPS) is an organic compound with the formula (HOC6H4)2SO2. It has two phenol functional groups on either side of a sulfonyl group. It is commonly used in curing fast-drying epoxy resin adhesives. It is classified as a bisphenol, and a close molecular analog of bisphenol A (BPA). BPS differentiates from BPA by possessing a sulfone group (SO2) as the central linker of the molecule instead of a dimethylmethylene group (C 2), which is the case of bisphenol A.

The International Organization for Standardization defines Engineered Nanomaterials, or ENMS, as materials with external dimensions between 1 and 100nm, the nanoscale, or having an internal surface structure at these dimensions. Nanoparticles can be both incidental and engineered. Incidental nanoparticles include particles from dust storms, volcanic eruptions, forest fires, and ocean water evaporation. Engineered nanoparticles (EMMs) are nanoparticles that are made for use in cosmetics or pharmaceuticals like ZnO and TiO2. They are also found from sources such as cigarette smoke and building demolition. Engineered nanoparticles have become increasingly important for many applications in consumer and industrial products, which has resulted in an increased presence in the environment. This proliferation has instigated a growing body of research into the effects of nanoparticles on the environment.

Per- and polyfluoroalkyl substances (PFASs) are a group of synthetic organofluorine chemical compounds that have multiple fluorine atoms attached to an alkyl chain. An early definition, from 2011, required that they contain at least one perfluoroalkyl moiety, –CnF2n+1–. Beginning in 2021, the Organisation for Economic Co-operation and Development (OECD) expanded their terminology, stating that "PFASs are defined as fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it), i.e. with a few noted exceptions, any chemical with at least a perfluorinated methyl group (–CF3) or a perfluorinated methylene group (–CF2–) is a PFAS."

The mercury cycle is a biogeochemical cycle influenced by natural and anthropogenic processes that transform mercury through multiple chemical forms and environments.

Tris(1,3-dichloroisopropyl)phosphate (TDCPP) is a chlorinated organophosphate. Organophosphate chemicals have a wide variety of applications and are used as flame retardants, pesticides, plasticizers, and nerve gases. TDCPP is structurally similar to several other organophosphate flame retardants, such as tris(2-chloroethyl) phosphate (TCEP) and tris(chloropropyl)phosphate (TCPP). TDCPP and these other chlorinated organophosphate flame retardants are all sometimes referred to as "chlorinated tris".
Galaxolide is a synthetic musk with a clean sweet musky floral woody odor used in fragrances. It is one of the musk components that perfume and cologne manufacturers use to add a musk odor to their products. Galaxolide was first synthesized in 1965, and used in the late 1960s in some fabric softeners and detergents. High concentrations were also incorporated in fine fragrances.
Heather M. Stapleton is an American environmental organic chemist and exposure scientist. She is the Ronie-Richele Garcia-Johnson Distinguished Professor at Duke University's Nicholas School of the Environment. During her tenure at Duke, Stapleton focused her research on identifying and understanding the uses of flame retardant chemicals in consumer products and evaluating the health impacts of exposures to those chemicals.

Decabromodiphenyl ethane is a chemical compound used as a brominated flame retardant. It was commercialised in the 1990s as an alternative for decabromodiphenyl ether, following safety concern over that compound. The two molecules are chemically very similar, which gives them a similar application profile. Decabromodiphenyl ethane is now also coming under regulatory pressure.

Bis(2-ethylhexyl)tetrabromophthalate (or TBPH), is a brominated phthalate derivative with the formula C24H34Br4O4 commonly used as a brominated flame retardant (BFR).