
A fibroblast is a type of biological cell typically with a spindle shape that synthesizes the extracellular matrix and collagen, produces the structural framework (stroma) for animal tissues, and plays a critical role in wound healing. Fibroblasts are the most common cells of connective tissue in animals.

Fibronectin is a high-molecular weight glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans.

Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, then, are metastases (mets). It is generally distinguished from cancer invasion, which is the direct extension and penetration by cancer cells into neighboring tissues.

In biology, the extracellular matrix (ECM), also called intercellular matrix (ICM), is a network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.
Stromal cells, or mesenchymal stromal cells, are differentiating cells found in abundance within bone marrow but can also be seen all around the body. Stromal cells can become connective tissue cells of any organ, for example in the uterine mucosa (endometrium), prostate, bone marrow, lymph node and the ovary. They are cells that support the function of the parenchymal cells of that organ. The most common stromal cells include fibroblasts and pericytes. The term stromal comes from Latin stromat-, "bed covering", and Ancient Greek στρῶμα, strôma, "bed".
Intravasation is the invasion of cancer cells through the basement membrane into a blood or lymphatic vessel. Intravasation is one of several carcinogenic events that initiate the escape of cancerous cells from their primary sites. Other mechanisms include invasion through basement membranes, extravasation, and colonization of distant metastatic sites. Cancer cell chemotaxis also relies on this migratory behavior to arrive at a secondary destination designated for cancer cell colonization.

72 kDa type IV collagenase also known as matrix metalloproteinase-2 (MMP-2) and gelatinase A is an enzyme that in humans is encoded by the MMP2 gene. The MMP2 gene is located on chromosome 16 at position 12.2.

Fibroblast activation protein alpha (FAP-alpha) also known as prolyl endopeptidase FAP is an enzyme that in humans is encoded by the FAP gene.

Periostin is a protein that in humans is encoded by the POSTN gene. Periostin functions as a ligand for alpha-V/beta-3 and alpha-V/beta-5 integrins to support adhesion and migration of epithelial cells.

Dermatopontin also known as tyrosine-rich acidic matrix protein (TRAMP) is a protein that in humans is encoded by the DPT gene. Dermatopontin is a 22-kDa protein of the noncollagenous extracellular matrix (ECM) estimated to comprise 12 mg/kg of wet dermis weight. To date, homologues have been identified in five different mammals and 12 different invertebrates with multiple functions. In vertebrates, the primary function of dermatopontin is a structural component of the ECM, cell adhesion, modulation of TGF-β activity and cellular quiescence). It also has pathological involvement in heart attacks and decreased expression in leiomyoma and fibrosis. In invertebrate, dermatopontin homologue plays a role in hemagglutination, cell-cell aggregation, and expression during parasite infection.
Pancreatic stellate cells (PaSCs) are classified as myofibroblast-like cells that are located in exocrine regions of the pancreas. PaSCs are mediated by paracrine and autocrine stimuli and share similarities with the hepatic stellate cell. Pancreatic stellate cell activation and expression of matrix molecules constitute the complex process that induces pancreatic fibrosis. Synthesis, deposition, maturation and remodelling of the fibrous connective tissue can be protective, however when persistent it impedes regular pancreatic function.
Metastatic breast cancer, also referred to as metastases, advanced breast cancer, secondary tumors, secondaries or stage IV breast cancer, is a stage of breast cancer where the breast cancer cells have spread to distant sites beyond the axillary lymph nodes. There is no cure for metastatic breast cancer; there is no stage after IV.

Mammary-type myofibroblastoma (MFB), also named mammary and extramammary myofibroblastoma, was first termed myofibrolastoma of the breast, or, more simply, either mammary myofibroblastoma (MMFB) or just myofibroblastoma. The change in this terminology occurred because the initial 1987 study and many subsequent studies found this tumor only in breast tissue. However, a 2001 study followed by numerous reports found tumors with the microscopic histopathology and other key features of mammary MFB in a wide range of organs and tissues. Further complicating the issue, early studies on MFB classified it as one of various types of spindle cell tumors that, except for MFB, were ill-defined. These other tumors, which have often been named interchangeably in different reports, are: myelofibroblastoma, benign spindle cell tumor, fibroma, spindle cell lipoma, myogenic stromal tumor, and solitary stromal tumor. Finally, studies suggest that spindle cell lipoma and cellular angiofibroma are variants of MFB. Here, the latter two tumors are tentatively classified as MFB variants but otherwise MFB is described as it is more strictly defined in most recent publications. The World Health Organization in 2020 classified mammary type myofibroblastoma tumors and myofibroblastoma tumors as separate tumor forms within the category of fibroblastic and myofibroblastic tumors.
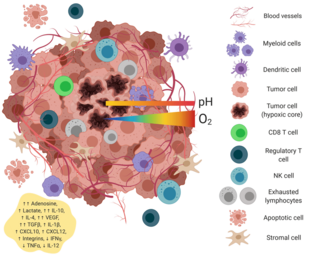
The tumor microenvironment is a complex ecosystem surrounding a tumor, composed of cancer cells, stromal tissue and the extracellular matrix. Mutual interaction between cancer cells and the different components of the tumor microenvironment support its growth and invasion in healthy tissues which correlates with tumor resistance to current treatments and poor prognosis. The tumor microenvironment is in constant change because of the tumor's ability to influence the microenvironment by releasing extracellular signals, promoting tumor angiogenesis and inducing peripheral immune tolerance, while the immune cells in the microenvironment can affect the growth and evolution of cancerous cells.
Oncomatryx Biopharma S. L. is a pharmaceutical biotechnology company that develops personalized treatments against invasive cancer as well as tests for its early detection. Established by Laureano Simón, PhD, Oncomatryx thus engages twofold in the fight against invasive kinds of cancer, such as pancreatic cancer or invasive breast cancer, all of which have high mortality rates.

Melanoma inhibitory activity protein 3 (MIA3), also known as transport and Golgi organization protein 1 (TANGO1), is a protein that in humans is encoded by the MIA3 gene on chromosome 1. It is ubiquitously expressed in many tissues and cell types. MIA3 localizes to the endoplasmic reticulum (ER) exit site, where it binds bulky cargo molecules such as collagens and creates mega transport carriers for the export of cargoes from the ER. This function suggests that it plays a role in assembly of extracellular matrix (ECM) and bone formation. MIA3 has been demonstrated to contribute to both tumor suppression and progression. The MIA3 gene also contains one of 27 loci associated with increased risk of coronary artery disease.. A TANGO1 like protein called TALI is expressed in liver and intestine and shown to be required for the export of bulky very Low density lipoproteins (VLDL) and chylomicrons. TANGO1 and TALI assemble into rings around COPII coats and this function is necessary for export of bulky cargoes. The discovery of TANGO1 and understanding its function has revealed that cargo export from the ER is not be vesicles but involves transient tunnels between the ER exit site and the next compartment of the secretory pathway. Biallelic Mutations in TANGO1 cause syndrome disease and complete loss of TANGO1 leads of defects in bone mineralization. These findings highlight the significance of TANGO1 in building and ER exit site, controlling the quantities and quality of cargo exported, which is necessary for life.Membrane permeant peptides of TANGO1 affect hyper collagen secretion in normal and cells of patients with scleroderma, and in a zebra fish model of wound healing. These findings raise the possibility of targeting TANGO1 to control skin scarring, wound healing and fibrosis.
A cancer-associated fibroblast (CAF) is a cell type within the tumor microenvironment that promotes tumorigenic features by initiating the remodelling of the extracellular matrix or by secreting cytokines. CAFs are a complex and abundant cell type within the tumour microenvironment; the number cannot decrease, as they are unable to undergo apoptosis.

Invasion is the process by which cancer cells directly extend and penetrate into neighboring tissues in cancer. It is generally distinguished from metastasis, which is the spread of cancer cells through the circulatory system or the lymphatic system to more distant locations. Yet, lymphovascular invasion is generally the first step of metastasis.

Edna "Eti" Cukierman is a Mexican biochemist who is a professor at the Fox Chase Cancer Center. She serves as co-director of the Marvin & Concetta Greenberg Pancreatic Cancer Institute. Her research investigates pancreatic cancer and the tumor microenvironment.
Invasion and metastasis are fundamental hallmarks of cancer, representing the ability of the cancer cells to spread from their site of origin to distant tissues and organs. These processes are central to cancer's lethality, accounting for the majority of cancer - related deaths, and marking an important barrier to effective treatment.
















