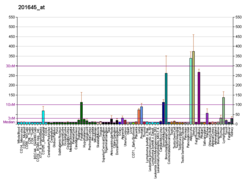
Versican is a large extracellular matrix proteoglycan that is present in a variety of human tissues. It is encoded by the VCAN gene.

CTGF, also known as CCN2 or connective tissue growth factor, is a matricellular protein of the CCN family of extracellular matrix-associated heparin-binding proteins. CTGF has important roles in many biological processes, including cell adhesion, migration, proliferation, angiogenesis, skeletal development, and tissue wound repair, and is critically involved in fibrotic disease and several forms of cancers.
Tenascins are extracellular matrix glycoproteins. They are abundant in the extracellular matrix of developing vertebrate embryos and they reappear around healing wounds and in the stroma of some tumors.

Integrin alpha-3 is a protein that in humans is encoded by the ITGA3 gene. ITGA3 is an integrin alpha subunit. Together with beta-1 subunit, it makes up half of the α3β1 integrin duplex that plays a role in neural migration and corticogenesis, acted upon by such factors as netrin-1 and reelin.

Disintegrin and metalloproteinase domain-containing protein 15 is an enzyme that in humans is encoded by the ADAM15 gene.

Laminin subunit gamma-2 is a protein that in humans is encoded by the LAMC2 gene.

Laminin subunit alpha-3 is a protein that in humans is encoded by the LAMA3 gene.

Receptor-type tyrosine-protein phosphatase F is an enzyme that in humans is encoded by the PTPRF gene.

Laminin subunit alpha-1 is a protein that in humans is encoded by the LAMA1 gene.

Laminin subunit beta-1 is a protein that in humans is encoded by the LAMB1 gene.

Alpha-7 integrin is a protein that in humans is encoded by the ITGA7 gene. Alpha-7 integrin is critical for modulating cell-matrix interactions. Alpha-7 integrin is highly expressed in cardiac muscle, skeletal muscle and smooth muscle cells, and localizes to Z-disc and costamere structures. Mutations in ITGA7 have been associated with congenital myopathies and noncompaction cardiomyopathy, and altered expression levels of alpha-7 integrin have been identified in various forms of muscular dystrophy.

Dentin matrix acidic phosphoprotein 1 is a protein that in humans is encoded by the DMP1 gene.

Fibulin-2 is a protein that in humans is encoded by the FBLN2 gene.
Latent-transforming growth factor beta-binding protein 1 is a protein that in humans is encoded by the LTBP1 gene.

Stromelysin-3 (SL-3) also known as matrix metalloproteinase-11 (MMP-11) is an enzyme that in humans is encoded by the MMP11 gene.

Collagen alpha-1(XIV) chain is a protein that in humans is encoded by the COL14A1 gene. It likely plays a role in collagen binding and cell-cell adhesion.

Collagen alpha-1(XIII) chain is a protein that in humans is encoded by the COL13A1 gene.

Integrin alpha-9 is a protein that in humans is encoded by the ITGA9 gene. Cytogenetic location: 3p22.2

Integrin beta-8 is a protein that in humans is encoded by the ITGB8 gene.

Integrin alpha-8 is a protein that in humans is encoded by the ITGA8 gene.