
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom bonded to two organyl groups. They have the general formula R−O−R′, where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

A solvent is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules, and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell.

Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. It is an isomer of another solvent, butanone.

Polyether ether ketone (PEEK) is a colourless organic thermoplastic polymer in the polyaryletherketone (PAEK) family, used in engineering applications. It was invented in November 1978 and brought to market in the early 1980s by part of Imperial Chemical Industries (ICI) that later became Victrex PLC.

The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. This reaction is important in the history of organic chemistry because it helped prove the structure of ethers.

Charles John Pedersen was an American organic chemist best known for discovering crown ethers and describing methods of synthesizing them during his entire 42-year career as a chemist for DuPont at DuPont Experimental Station in Wilmington, Delaware, and at DuPont's Jackson Laboratory in Deepwater, New Jersey. Often associated with Reed McNeil Izatt, Pedersen also shared the Nobel Prize in Chemistry in 1987 with Donald J. Cram and Jean-Marie Lehn. He is the one of three Nobel Prize laureates born in Korea, along with Peace Prize laureate Kim Dae-jung and Literature laureate Han Kang.

In organic chemistry, crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups (R−O−R’). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., −CH2CH2O−. Important members of this series are the tetramer (n = 4), the pentamer (n = 5), and the hexamer (n = 6). The term "crown" refers to the resemblance between the structure of a crown ether bound to a cation, and a crown sitting on a person's head. The first number in a crown ether's name refers to the number of atoms in the cycle, and the second number refers to the number of those atoms that are oxygen. Crown ethers are much broader than the oligomers of ethylene oxide; an important group are derived from catechol.
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of catalysis and can act through homogeneous catalysis or heterogeneous catalysis methods depending on the catalyst used. Ionic reactants are often soluble in an aqueous phase but insoluble in an organic phase in the absence of the phase-transfer catalyst. The catalyst functions like a detergent for solubilizing the salts into the organic phase. Phase-transfer catalysis refers to the acceleration of the reaction upon the addition of the phase-transfer catalyst.
Chiral column chromatography is a variant of column chromatography that is employed for the separation of chiral compounds, i.e. enantiomers, in mixtures such as racemates or related compounds. The chiral stationary phase (CSP) is made of a support, usually silica based, on which a chiral reagent or a macromolecule with numerous chiral centers is bonded or immobilized.

18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1). The point group of 18-crown-6 is S6. The dipole moment of 18-crown-6 is solvent- and temperature-dependent. Below 25 °C, the dipole moment of 18-crown-6 is 2.76 ± 0.06 D in cyclohexane and 2.73 ± 0.02 in benzene. The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen.
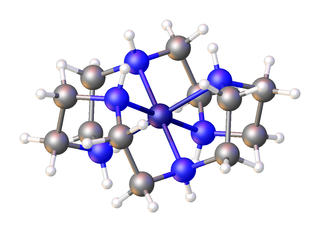
In organic chemistry, an aza-crown ether is an aza analogue of a crown ether. That is, it has a nitrogen atom in place of each oxygen atom around the ring. While the parent crown ethers have the formulae (CH2CH2O)n, the parent aza-crown ethers have the formulae (CH2CH2NH)n, where n = 3, 4, 5, 6. Well-studied aza crowns include triazacyclononane, cyclen, and hexaaza-18-crown-6.

12-Crown-4, also called 1,4,7,10-tetraoxacyclododecane and lithium ionophore V, is a crown ether with the formula C8H16O4. It is a cyclic tetramer of ethylene oxide which is specific for the lithium cation.

15-Crown-5 is a crown ether with the formula (C2H4O)5. It is a cyclic pentamer of ethylene oxide that forms complex with various cations, including sodium (Na+) and potassium (K+); however, it is complementary to Na+ and thus has a higher selectivity for Na+ ions.
Bis(chloroethyl) ether is an organic compound with the formula O(CH2CH2Cl)2. It is an ether with two 2-chloroethyl substituents. It is a colorless liquid with the odor of a chlorinated solvent.
William Clark Still is an American organic chemist. As a distinguished professor at Columbia University, Clark Still made significant contributions to the field of organic chemistry, particularly in the areas of natural product synthesis, reaction development, conformational analysis, macrocyclic stereocontrol, and computational chemistry. Still and coworkers also developed the purification technique known as flash column chromatography which is widely used for the purification of organic compounds.
In coordination chemistry, a macrocyclic ligand is a macrocyclic ring having at least nine atoms and three or more donor sites that serve as ligands. Crown ethers and porphyrins are prominent examples. Macrocyclic ligands often exhibit high affinity for metal ions, the macrocyclic effect.

21-Crown-7 is an organic compound with the formula [C2H4O]7 and the IUPAC name 1,4,7,10,13,16,19-heptaoxacycloheneicosane. Similar to other crown ethers, 21-crown-7 functions as a ligand for certain metal cations, with a specific affinity for caesium cations. The dipole moment of 21-crown-7 varies depending on the solvent and temperatures.

In organic chemistry, thia-crown ethers are organosulfur compounds which are the thia analogues of crown ethers. That is, they have a sulfur atom in place of each oxygen atom around the ring. While the parent crown ethers have the formulae (CH2CH2O)n, the parent thia-crown ethers have the formulae (CH2CH2S)n, where n = 3, 4, 5, 6. They have trivial names "x-ane-Sy", where x and y are the number of atoms in the ring and the number of those atoms that are sulfur, respectively. Thia-crown ethers exhibit affinities for transition metals.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.
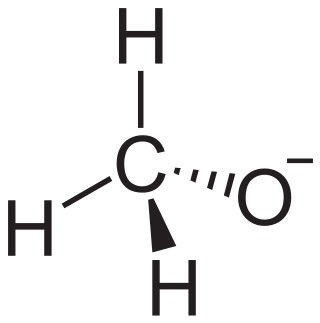
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as RO−, where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis. Transition metal alkoxides are widely used for coatings and as catalysts.


![Structure of [Na(dibenzo-18-c-6)] , as found in the FeCl4 salt. Na(dibenzo18-c-6)FeCl4 (TITWIC).png](//upload.wikimedia.org/wikipedia/commons/thumb/d/df/Na%28dibenzo18-c-6%29FeCl4_%28TITWIC%29.png/220px-Na%28dibenzo18-c-6%29FeCl4_%28TITWIC%29.png)

![Structure of [Na(dibenzo-18-c-6)] , as found in the FeCl4 salt. Na(dibenzo18-c-6)FeCl4 (TITWIC).png](http://upload.wikimedia.org/wikipedia/commons/thumb/d/df/Na%28dibenzo18-c-6%29FeCl4_%28TITWIC%29.png/220px-Na%28dibenzo18-c-6%29FeCl4_%28TITWIC%29.png)














