A carbohydrate is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 and thus with the empirical formula Cm(H2O)n, which does not mean the H has covalent bonds with O. However, not all carbohydrates conform to this precise stoichiometric definition, nor are all chemicals that do conform to this definition automatically classified as carbohydrates.

In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.

Otto Stern was a German-American physicist and Nobel laureate in physics. He was the second most nominated physicist for a Nobel Prize, with 82 nominations in the years 1925–1945, ultimately winning in 1943.
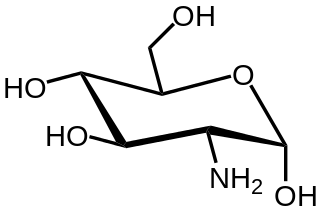
In organic chemistry, an amino sugar is a sugar molecule in which a hydroxyl group has been replaced with an amine group. More than 60 amino sugars are known, with one of the most abundant being N-acetyl-D-glucosamine, which is the main component of chitin.
Galactokinase is an enzyme (phosphotransferase) that facilitates the phosphorylation of α-D-galactose to galactose 1-phosphate at the expense of one molecule of ATP. Galactokinase catalyzes the second step of the Leloir pathway, a metabolic pathway found in most organisms for the catabolism of α-D-galactose to glucose 1-phosphate. First isolated from mammalian liver, galactokinase has been studied extensively in yeast, archaea, plants, and humans.
The Ferrier rearrangement is an organic reaction that involves a nucleophilic substitution reaction combined with an allylic shift in a glycal. It was discovered by the carbohydrate chemist Robert J. Ferrier.

Fucose is a hexose deoxy sugar with the chemical formula C6H12O5. It is found on N-linked glycans on the mammalian, insect and plant cell surface. Fucose is the fundamental sub-unit of the seaweed polysaccharide fucoidan. The α(1→3) linked core of fucoidan is a suspected carbohydrate antigen for IgE-mediated allergy.

Fucitol, also known as L-fucitol, 1-deoxy-L-galactitol, and (2R,3S,4R,5S)-hexane-1,2,3,4,5-pentol, is a sugar alcohol derived from fucoidan which is found in the North Atlantic seaweed Fucus vesiculosus or by the reduction of fucose.
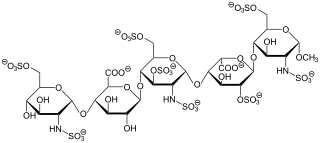
Fondaparinux is an anticoagulant medication chemically related to low molecular weight heparins. It is marketed by Viatris. A generic version developed by Alchemia is marketed within the US by Dr. Reddy's Laboratories.

[18F]Fluorodeoxyglucose (INN), or fluorodeoxyglucose F 18, also commonly called fluorodeoxyglucose and abbreviated [18F]FDG, 2-[18F]FDG or FDG, is a radiopharmaceutical, specifically a radiotracer, used in the medical imaging modality positron emission tomography (PET). Chemically, it is 2-deoxy-2-[18F]fluoro-D-glucose, a glucose analog, with the positron-emitting radionuclide fluorine-18 substituted for the normal hydroxyl group at the C-2 position in the glucose molecule.

Quercitrin is a glycoside formed from the flavonoid quercetin and the deoxy sugar rhamnose.

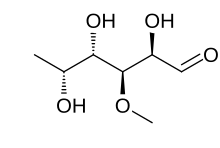
Deoxy sugars are sugars that have had a hydroxyl group replaced with a hydrogen atom.

In enzymology, a dTDP-4-dehydrorhamnose 3,5-epimerase is an enzyme that catalyzes the chemical reaction
The enzyme dTDP-glucose 4,6-dehydratase (EC 4.2.1.46) catalyzes the chemical reaction

The enzyme GDP-mannose 4,6-dehydratase (EC 4.2.1.47) catalyzes the chemical reaction

Desosamine is a 3-(dimethylamino)-3,4,6-trideoxyhexose found in certain macrolide antibiotics such as the commonly prescribed erythromycin, azithromycin, clarithroymcin, methymycin, narbomycin, oleandomycin, picromycin and roxithromycin. As the name suggests, these macrolide antibiotics contain a macrolide or lactone ring and they are attached to the ring Desosamine which is crucial for bactericidal activity. The biological action of the desosamine-based macrolide antibiotics is to inhibit the bacterial ribosomal protein synthesis. These antibiotics which contain Desosamine are widely used to cure bacterial-causing infections in human respiratory system, skin, muscle tissues, and urethra.
A chemical glycosylation reaction involves the coupling of a glycosyl donor, to a glycosyl acceptor forming a glycoside. If both the donor and acceptor are sugars, then the product is an oligosaccharide. The reaction requires activation with a suitable activating reagent. The reactions often result in a mixture of products due to the creation of a new stereogenic centre at the anomeric position of the glycosyl donor. The formation of a glycosidic linkage allows for the synthesis of complex polysaccharides which may play important roles in biological processes and pathogenesis and therefore having synthetic analogs of these molecules allows for further studies with respect to their biological importance.
Lipid IVA 4-amino-4-deoxy-L-arabinosyltransferase is an enzyme with systematic name 4-amino-4-deoxy-alpha-L-arabinopyranosyl ditrans, octacis-undecaprenyl phosphate:lipid IVA 4-amino-4-deoxy-L-arabinopyranosyltransferase. This enzyme catalyses the following chemical reaction
Lipid IVA 3-deoxy-D-manno-octulosonic acid transferase is an enzyme with systematic name CMP-3-deoxy-D-manno-oct-2-ulosonate:lipid IVA 3-deoxy-D-manno-oct-2-ulosonate transferase. This enzyme catalyses the following chemical reaction
(KDO)2-lipid IVA (2-8) 3-deoxy-D-manno-octulosonic acid transferase is an enzyme with systematic name CMP-3-deoxy-D-manno-oct-2-ulosonate:(KDO)2-lipid IVA 3-deoxy-D-manno-oct-2-ulosonate transferase . This enzyme catalyses the following chemical reaction