
A grape is a fruit, botanically a berry, of the deciduous woody vines of the flowering plant genus Vitis.

Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural phenol, and a phytoalexin produced by several plants in response to injury or when the plant is under attack by pathogens, such as bacteria or fungi. Sources of resveratrol in food include the skin of grapes, blueberries, raspberries, mulberries, and peanuts.

Vitis rotundifolia, or muscadine, is a grapevine species native to the southeastern and south-central United States. The growth range extends from Florida to New Jersey coast, and west to eastern Texas and Oklahoma. It has been extensively cultivated since the 16th century. The plants are well-adapted to their native warm and humid climate; they need fewer chilling hours than better known varieties, and thrive in summer heat.
Calorie restriction mimetics (CRM), also known as energy restriction mimetics, are a hypothetical class of dietary supplements or drug candidates that would, in principle, mimic the substantial anti-aging effects that calorie restriction (CR) has on many laboratory animals and humans. CR is defined as a reduction in calorie intake of 20% to 50% without incurring malnutrition or a reduction in essential nutrients. An effective CRM would alter the key metabolic pathways involved in the effects of CR itself, leading to preserved youthful health and longer lifespan without the need to reduce food intake. The term was coined by Lane, Ingram, Roth of the National Institute on Aging in a seminal 1998 paper in the Journal of Anti-Aging Medicine, the forerunner of Rejuvenation Research. A number of genes and pathways have been shown to be involved the actions of CR in model organisms and these represent attractive targets for drug discovery and for developing CRM. However, no effective CRM have been identified to date.
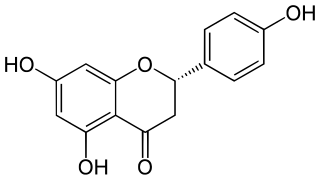
Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs.

The health effects of wine are mainly determined by its active ingredient alcohol. Preliminary studies found that drinking small quantities of wine, particularly of red wine, may be associated with a decreased risk of cardiovascular diseases, cognitive decline, stroke, diabetes mellitus, metabolic syndrome, and early death. Other studies found no such effects.

Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Most stilbenoids are produced by plants, and the only known exception is the antihelminthic and antimicrobial stilbenoid, 2-isopropyl-5-[(E)-2-phenylvinyl]benzene-1,3-diol, biosynthesized by the Gram-negative bacterium Photorhabdus luminescens.
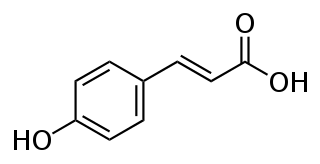
p-Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers of coumaric acid—o-coumaric acid, m-coumaric acid, and p-coumaric acid—that differ by the position of the hydroxy substitution of the phenyl group. p-Coumaric acid is the most abundant isomer of the three in nature. p-Coumaric acid exists in two forms trans-p-coumaric acid and cis-p-coumaric acid.
The Julia olefination (also known as the Julia–Lythgoe olefination) is the chemical reaction used in organic chemistry of phenyl sulfones (1) with aldehydes (or ketones) to give alkenes (olefins)(3) after alcohol functionalization and reductive elimination using sodium amalgam or SmI2. The reaction is named after the French chemist Marc Julia.

Piceid is a stilbenoid glucoside and is a major resveratrol derivative in grape juices. It can be found in the bark of Picea sitchensis. It can also be isolated from Reynoutria japonica, the Japanese knotweed.

Piceatannol is a stilbenoid, a type of phenolic compound.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

ε-Viniferin is a naturally occurring phenol, belonging to the stilbenoids family. It is a resveratrol dimer.

In biochemistry, naturally occurring phenols refers to phenol functional group that is found in natural products. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

Astringin is a stilbenoid, the 3-β-D-glucoside of piceatannol. It can be found in the bark of Picea sitchensis or Picea abies.

Wine is a complex mixture of chemical compounds in a hydro-alcoholic solution with a pH around 4.

trans-Resveratrol-3-O-glucuronide is a metabolite of resveratrol and trans-resveratrol-3-O-glucoside (piceid).

δ-Viniferin is a resveratrol dehydrodimer. It is an isomer of epsilon-viniferin. It can be isolated from stressed grapevine leaves. It is also found in plant cell cultures and wine. It can also be found in Rheum maximowiczii.

Dihydrostilbenoids (bibenzyls) are natural phenols formed from the dihydrostilbene (bibenzyl) backbone.
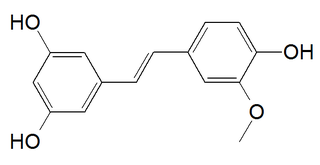
Isorhapontigenin is a tetrahydroxylated stilbenoid with a methoxy group. It is an isomer of rhapontigenin and an analog of resveratrol. It is found in the Chinese herb Gnetum cleistostachyum, in Gnetum parvifolium and in the seeds of the palm Aiphanes aculeata.

















