
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. It is radioactive, poisonous, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous.

The Wharton olefin synthesis or the Wharton reaction is a chemical reaction that involves the reduction of α,β-epoxy ketones using hydrazine to give allylic alcohols. This reaction, introduced in 1961 by P. S. Wharton, is an extension of the Wolff–Kishner reduction. The general features of this synthesis are: 1) the epoxidation of α,β-unsaturated ketones is achieved usually in basic conditions using hydrogen peroxide solution in high yield; 2) the epoxy ketone is treated with 2–3 equivalents of a hydrazine hydrate in presence of substoichiometric amounts of acetic acid. This reaction occurs rapidly at room temperature with the evolution of nitrogen and the formation of an allylic alcohol. It can be used to synthesize carenol compounds. Wharton's initial procedure has been improved.

Pederin is a vesicant toxic amide with two tetrahydropyran rings, found in the haemolymph of the beetle genus Paederus, including the Nairobi fly, belonging to the family Staphylinidae. It was first characterized by processing 25 million field-collected P. fuscipes. It makes up approximately 0.025% of an insects weight.

Betulin is an abundant, naturally occurring triterpene. It is commonly isolated from the bark of birch trees. It forms up to 30% of the dry weight of silver birch bark. It is also found in birch sap. Inonotus obliquus and red alder also contain betulin.
The Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product. The mechanism of the reaction was proposed in its original disclosure by A.G. Brook with further evidence later supplied by George M. Rubottom. After a Prilezhaev-type oxidation of the silyl enol ether with the peroxyacid to form the siloxy oxirane intermediate, acid-catalyzed ring-opening yields an oxocarbenium ion. This intermediate then participates in a 1,4-silyl migration to give an α-siloxy carbonyl derivative that can be readily converted to the α-hydroxy carbonyl compound in the presence of acid, base, or a fluoride source.

Oenanthotoxin is a toxin extracted from hemlock water-dropwort and other plants of the genus Oenanthe. It is a central nervous system poison, and acts as a noncompetitive antagonist of the neurotransmitter gamma-aminobutyric acid. A case has been made for the presence of this toxin in local Oenanthe species playing a causative role in euthanasia in ancient Sardinia. It was crystallized in 1949 by Clarke and co-workers. It is structurally closely related to the toxins cicutoxin and carotatoxin. Oenanthotoxin is a C17 polyacetylene isomer of cicutoxin.

Acetogenins are a class of polyketide natural products found in plants of the family Annonaceae. They are characterized by linear 32- or 34-carbon chains containing oxygenated functional groups including hydroxyls, ketones, epoxides, tetrahydrofurans and tetrahydropyrans. They are often terminated with a lactone or butenolide. Over 400 members of this family of compounds have been isolated from 51 different species of plants. Many acetogenins are characterized by neurotoxicity.

Xanthohumol is a natural product found in the female inflorescences of Humulus lupulus, also known as hops. This compound is also found in beer and belongs to a class of compounds that contribute to the bitterness and flavor of hops. Xanthohumol is a prenylated chalconoid, biosynthesized by a type III polyketide synthase (PKS) and subsequent modifying enzymes.

A xanthonoid is a chemical natural phenolic compound formed from the xanthone backbone. Many members of the Clusiaceae contain xanthonoids.
Bohemic acid is a mixture of chemical compounds which is obtained through fermentation by actinobacteria species in the genus Actinosporangium (Actinoplanaceae). The name honors the Puccini opera La Bohème and many individual components of the acid carry the names of characters from La Bohème. Most of those components are antitumor agents and anthracycline antibiotics active against Gram-positive bacteria.

δ-Viniferin is a resveratrol dehydrodimer. It is an isomer of epsilon-viniferin. It can be isolated from stressed grapevine leaves. It is also found in plant cell cultures and wine. It can also be found in Rheum maximowiczii.

Notholaena standleyi, also known as star cloak fern and Standley's cloak fern, is a fern that is native to the United States and Mexico. It is a member of the genus Notholaena, which is part of the subfamily Cheilanthoideae of family Pteridaceae. It is distinguished by the pentagonal shape formed by the blades of its frond, a property other members of Notholaena do not possess.
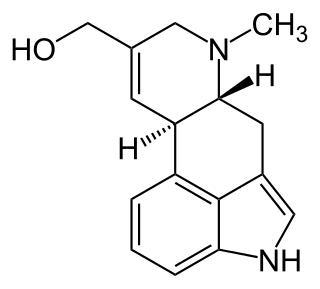
Elymoclavine is an ergot alkaloid. It can be produced from C. fusiformis from Pennisetum typhoideum. It is a precursor in the biosynthesis of D-(+)-lysergic acid. Ergot alkaloids are natural products derived from L-tryptophan. They are often toxic for humans and animals. Despite that they are also well known for their pharmacological activities.
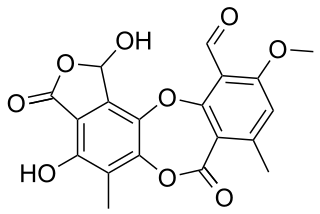
Stictic acid is an aromatic organic compound, a product of secondary metabolism in some species of lichens.

Secalonic acids are a group of chiral dimeric tetrahydroxanthones closely related to ergoflavin and ergochrysin A that are collectively called ergochromes and belong to a class of mycotoxins initially isolated as major ergot pigments from the fungi Claviceps purpurea that grows parasitically on rye grasses. From early times and particularly in medieval Europe the consumption of grains containing ergot has repeatedly lead to mass poisonings known as ergotism which was caused by toxic ergot alkaloids and mycotoxins such as the ergochromes, due to contamination of flour by C. purpurea. A cluster of genes responsible for the synthesis of secalonic acids in C. purpurea has been identified. Secalonic acid D the enantiomer of secalonic acid A is a major environmental toxin, isolated from the fungus Penicillium oxalicum, and is a major microbial contaminant of freshly-harvested corn which causes toxicity through contamination of foodstuffs.
Naphthomycins are a group of closely related antimicrobial chemical compounds isolated from Streptomyces. They are considered a subclass of ansamycins.
Penicillium chermesinum is an anamorph fungus species of the genus of Penicillium which was isolated from soil from Nova Scotia in Canada.Penicillium chermesinum produces plastatin, luteosporin, xanthomegnin, azaphilones, p-terphenyls and costaclavine.
Penicillium paneum is a species of fungus in the genus Penicillium which can spoil cereal grains. Penicillium paneum produces 1-Octen-3-ol and penipanoid A, penipanoid B, penipanoid C, patulin and roquefortine C
Penicillium sumatrense is a species of fungus in the genus Penicillium which was isolated from the rhizosphere of the plant Lumnitzera racemosa. Penicillium sumatrense produces sumalarin A, sumalarin B, sumalarin C
Streptomyces diastaticus is an alkaliphilic and thermophilic bacterium species from the genus of Streptomyces. Streptomyces diastaticus produces oligomycin A, oligomycin C, rimocidin and the leukotriene-A4 hydrolase-inhibitor 8(S)-amino-2(R)-methyl-7-oxononanoic acid. Streptomyces diastaticus also produces gougerotin and diastaphenazine and the antibiotic ruticin.













