In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.

Primula veris, the cowslip, common cowslip, or cowslip primrose, is a herbaceous perennial flowering plant in the primrose family Primulaceae. The species is native throughout most of temperate Europe and western Asia, and although absent from more northerly areas including much of northwest Scotland, it reappears in northernmost Sutherland and Orkney and in Scandinavia. This species frequently hybridizes with other Primulas such as the common primrose Primula vulgaris to form false oxlip which is often confused with true oxlip, a much rarer plant.
A calixarene is a macrocycle or cyclic oligomer based on a hydroxyalkylation product of a phenol and an aldehyde.

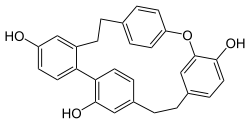
Cavicularin is a natural phenolic secondary metabolite isolated from the liverwort Cavicularia densa. This macrocycle is unusual because it was the first compound isolated from nature displaying optical activity solely due to the presence of planar chirality and axial chirality. The specific rotation for (+)-cavicularin is +168.2°. It is also a very strained molecule. The para-substituted phenol ring is bent about 15° out of planarity, adopting a somewhat boat-like geometry. This type of angle strain in aromatic compounds is normally reserved for synthetic cyclophanes.

Macrocycles are often described as molecules and ions containing a twelve or more membered ring. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.

A cyclophane is a hydrocarbon consisting of an aromatic unit and an aliphatic chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known. Cyclophanes are well-studied in organic chemistry because they adopt unusual chemical conformations due to build-up of strain.
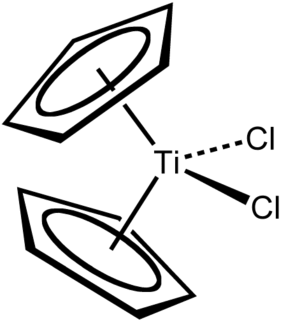
Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.

Phenanthrenoids are chemical compounds formed with a phenanthrene backbone. These compounds occur naturally in plants, although they can also be synthesized.

A boronic acid is a compound related to boric acid in which one of the three hydroxyl groups is replaced by an alkyl or aryl group. As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic acids, etc.. The pKa of a boronic acid is ~9, but they can form tetrahedral boronate complexes with pKa ~7. They are occasionally used in the area of molecular recognition to bind to saccharides for fluorescent detection or selective transport of saccharides across membranes.

Organotitanium compounds in organometallic chemistry contain carbon-to-titanium chemical bonds. Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry and are involved in major industrial processes.

Diphenylphosphine, also known as diphenylphosphane, is an organophosphorus compound with the formula (C6H5)2PH. This foul-smelling, colorless liquid is easily oxidized in air. It is a precursor to organophosphorus ligands for use as catalysts.

Perrottetinene is a naturally occurring cannabinoid compound found in liverworts from the genus Radula native to Japan, New Zealand and Costa Rica, namely Radula perrottetii, Radula marginata and Radula laxiramea, along with a number of similar compounds. Its chemical structure closely resembles that of THC, the main active component of marijuana. The absolute configuration of perrottetinene was established in 2008 by an enantioselective total synthesis. In 2018, a study showed that perrottetinene is moderately psychoactive through activation of the cannabinoid receptor 1. The same study also reported reduced prostaglandin D2 and E2 brain concentrations in mice after perrottetinene administration.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal with anionic bis(trimethylsilyl)amide ligands and are part of a broader category of metal amides.

Bibenzyl is the organic compound with the formula (C6H5CH2)2. It can be viewed as a derivative of ethane in which one phenyl group is bonded to each carbon atom. It is a colorless solid.

Dihydrostilbenoids (bibenzyls) are natural phenols formed from the dihydrostilbene (bibenzyl) backbone.

2,6-Diacetylpyridine is an organic compound with the formula C5H3N(C(O)CH3)2. It is a white solid that is soluble in organic solvents. It is a disubstituted pyridine. It is a precursor to ligands in coordination chemistry.
Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C–H bonds and are therefore useful reactions for carbon–hydrogen bond activation. Metal-catalyzed C–H borylation reactions utilize transition metals to directly convert a C–H bond into a C–B bond. This route can be advantageous compared to traditional borylation reactions by making use of cheap and abundant hydrocarbon starting material, limiting prefunctionalized organic compounds, reducing toxic byproducts, and streamlining the synthesis of biologically important molecules. Boronic acids, and boronic esters are common boryl groups incorporated into organic molecules through borylation reactions. Boronic acids are trivalent boron-containing organic compounds that possess one alkyl substituent and two hydroxyl groups. Similarly, boronic esters possess one alkyl substituent and two ester groups. Boronic acids and esters are classified depending on the type of carbon group (R) directly bonded to boron, for example alkyl-, alkenyl-, alkynyl-, and aryl-boronic esters. The most common type of starting materials that incorporate boronic esters into organic compounds for transition metal catalyzed borylation reactions have the general formula (RO)2B-B(OR)2. For example, bis(pinacolato)diboron (B2Pin2), and bis(catecholato)diborane (B2Cat2) are common boron sources of this general formula.
William Clark Still is an American organic chemist. As a distinguished professor at Columbia University, Clark Still made significant contributions to the field of organic chemistry, particularly in the areas of natural product synthesis, reaction development, conformational analysis, macrocyclic stereocontrol, and computational chemistry. Still and coworkers also developed the purification technique known as flash column chromatography which is widely used for the purification of organic compounds.
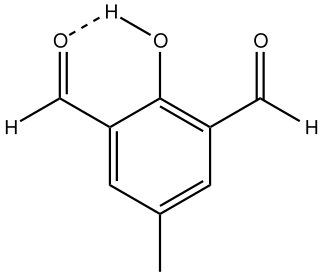
Diformylcresol is an organic compound with the formula CH3C6H2(CHO)2H. The 2,6-diformyl derivative of p-cresol is the most common isomer and is a white solid at room temperature.