
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.

Congo red is an organic compound, the sodium salt of 3,3′-([1,1′-biphenyl]-4,4′-diyl)bis(4-aminonaphthalene-1-sulfonic acid). It is an azo dye. Congo red is water-soluble, yielding a red colloidal solution; its solubility is greater in organic solvents. The use of Congo red in the textile industry has long been abandoned, primarily because of its carcinogenic properties, but it is still used for histological staining.

Azo compounds are organic compounds bearing the functional group diazenyl.

Sudan I is an organic compound typically classified as an azo dye. It is an orange-red solid, used to color waxes, oils, petrol, solvents, and polishes. Historically, Sudan I used to serve as a food coloring agent, notably for curry powder and chili powder. However, along with its derivatives Sudan III and Sudan IV, the compound has been banned in many countries due to its classification as a category 3 carcinogenic hazard by the International Agency for Research on Cancer. Nevertheless, Sudan I remains valuable as a coloring reagent for non-food-related uses, such as in the formulation of orange-colored smoke.

An acid dye is a dye that is typically applied to a textile at low pH. They are mainly used to dye wool, not cotton fabrics. Some acid dyes are used as food colorants, and some can also be used to stain organelles in the medical field.

Mordant red 19 is an organic compound with the chemical formula C16H13ClN4O5S. It is classified as an azo dye.

Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents.
In organic chemistry, an azo coupling is an reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile, and the activated carbon, serves as a nucleophile. Classical coupling agents are phenols and naphthols. Usually the diazonium reagent attacks at the para position of the coupling agent. When the para position is occupied, coupling occurs at a ortho position, albeit at a slower rate.
Trypan blue is an azo dye. It is a direct dye for cotton textiles. In biosciences, it is used as a vital stain to selectively colour dead tissues or cells blue.
A substantive dye or direct dye is a dye that adheres to its substrate, typically a textile, by non-ionic forces.
Solvent Black 3 is an azo dye. It is a non-fluorescent, relatively thermostable lysochrome diazo dye used for staining of neutral triglycerides and lipids on frozen sections and some lipoproteins on paraffin sections. It has the appearance of a dark brown to black powder with maximum absorption at 596–605 nm and melting point 120–124 °C. It stains blue-black.
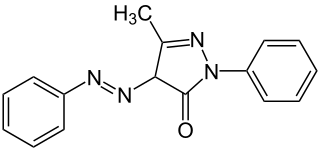
Sudan Yellow 3G, also known as Solvent Yellow 16, C.I. disperse yellow and C.I. 12700, is a yellow azo dye. It is soluble in fats and oils.
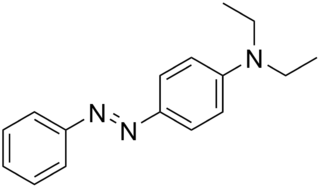
Solvent Yellow 56 is the organic compound N,N-diethyl-p-(phenylazo)aniline. It is an azo dye, which has the appearance of a reddish yellow powder. Its EINECS number is 219-616-8. Its structure is similar to Solvent Yellow 124, which used as a fuel dye in European Union, and to Aniline Yellow.

Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry.

Acid Orange 7, also known as 2-naphthol orange is an azo dye. It is used for dyeing wool.

Direct Blue 1 is an organic compound that is one of many azo dyes. This salt is used as a substantive dye for textiles with high contents of cellulose, i.e. cotton. It is prepared by the azo coupling of the aminonaphthalene and diazotized derivative of o-dianisidine.

Acid red 88 is an azo dye. Due to its intense colour, solid samples appear almost black. It is used to dye cotton textiles red. A closely related acid dye is Acid Red 13.
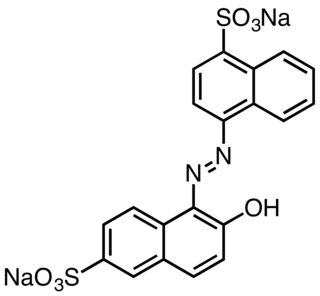
Acid Red 13 is an azo dye. It is produced as a sodium salt. Solid samples appear dark red.

N-Ethyl-N-(2-chloroethyl)aniline is the organic compound with the formula C6H5N(Et)(CH2CH2Cl) (Et = ethyl). It is a low-melting colorless solid that is an alkylating agent. The compound is a precursor to several cationic azo dyes via reaction of the chloroethyl group with tertiary amines or pyridine followed by azo coupling. Examples of derived dyes include C. I. Basic Red 18, Maxilon Red 2GL, and Yoracryl Red 2G.
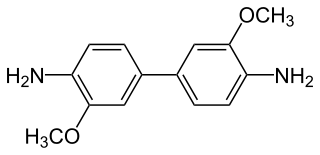
o-Dianisidine is an organic compound with the formula [(CH3O)(H2N)C6H3]2. A colorless or white solid, it is a bifunctional compound derived via the benzidine rearrangement from o-anisidine.
















