
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.

Tacticity is the relative stereochemistry of adjacent chiral centers within a macromolecule. The practical significance of tacticity rests on the effects on the physical properties of the polymer. The regularity of the macromolecular structure influences the degree to which it has rigid, crystalline long range order or flexible, amorphous long range disorder. Precise knowledge of tacticity of a polymer also helps understanding at what temperature a polymer melts, how soluble it is in a solvent and its mechanical properties.
A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:

In chemistry, an enantiomer, also known as an optical isomer, is one of two stereoisomers that are mirror images of each other that are non-superposable, much as one's left and right hands are have the same shape except for being reversed along one axis. A single chiral atom or similar structural feature in a compound causes that compound to have two possible structures which are non-superposable, each a mirror image of the other. Each member of the pair is termed an enantiomorph ; the structural property is termed enantiomerism. The presence of multiple chiral features in a given compound increases the number of geometric forms possible, though there may still be some perfect-mirror-image pairs.

In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products are strongly dependent on the temperature and presence of catalysts. Cracking is the breakdown of a large alkane into smaller, more useful alkenes. Simply put, hydrocarbon cracking is the process of breaking a long-chain of hydrocarbons into short ones. This process requires high temperatures and high pressure.
In a molecule, a stereocenter is a particular instance of a stereogenic element that is geometrically a point. A stereocenter or stereogenic center is any point in a molecule, though not necessarily an atom, bearing groups, such that an interchanging of any two groups leads to a stereoisomer. The term stereocenter was introduced in 1984 by Kurt Mislow and Jay Siegel. A chiral center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superimposable on its mirror image. The concept of a chiral center generalizes the concept of an asymmetric carbon atom such that an interchanging of any two groups gives rise to an enantiomer. In organic chemistry, a chiral center usually refers to a carbon, phosphorus, or sulfur atom, though it is also possible for other atoms to be chiral centers, especially in areas of organometallic and inorganic chemistry.
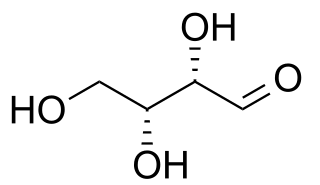
Diastereomers are a type of a stereoisomer. Diasteoreomers are defined as non-mirror image non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two.

A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereogenic centers, the molecule is not chiral. A meso compound is "superposable" on its mirror image. Two objects can be superposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter.

Polybutadiene [butadiene rubber BR] is a synthetic rubber. Polybutadiene rubber is a polymer formed from the polymerization of the monomer 1,3-butadiene. Polybutadiene has a high resistance to wear and is used especially in the manufacture of tires, which consumes about 70% of the production. Another 25% is used as an additive to improve the toughness of plastics such as polystyrene and acrylonitrile butadiene styrene (ABS). Polybutadiene rubber accounted for about a quarter of total global consumption of synthetic rubbers in 2012. It is also used to manufacture golf balls, various elastic objects and to coat or encapsulate electronic assemblies, offering high electrical resistivity.

Hot melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is tacky when hot, and solidifies in a few seconds to one minute. Hot melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.
Silicone rubber is an elastomer composed of silicone—itself a polymer—containing silicon together with carbon, hydrogen, and oxygen. Silicone rubbers are widely used in industry, and there are multiple formulations. Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost. Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from -67 °F to 572 °F while still maintaining its useful properties. Due to these properties and its ease of manufacturing and shaping, silicone rubber can be found in a wide variety of products, including: voltage line insulators, automotive applications; cooking, baking, and food storage products; apparel such as undergarments, sportswear, and footwear; electronics; medical devices and implants; and in home repair and hardware with products such as silicone sealants.

α-Tocopherol is a type of vitamin E. It has E number "E307". Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals and a hydrophobic side chain which allows for penetration into biological membranes. Compared to the others, α-tocopherol is preferentially absorbed and accumulated in humans.

A chiral derivatizing agent (CDA) also known as a chiral resolving reagent, is a chiral auxiliary used to convert a mixture of enantiomers into diastereomers in order to analyze the quantities of each enantiomer present within the mix. Analysis can be conducted by spectroscopy or by chromatography. The use of chiral derivatizing agents has declined with the popularization of chiral HPLC. Besides analysis, chiral derivatization is also used for chiral resolution, the actual physical separation of the enantiomers.
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972.
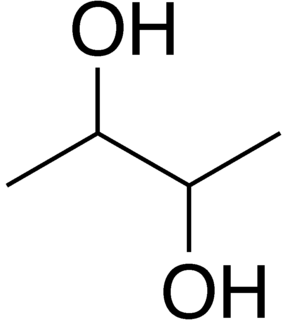
2,3-Butanediol is the organic compound with the formula (CH3CHOH)2. It is classified as a vic-diol (glycol). It exists as three stereoisomers, a chiral pair and the meso isomer. All are colorless liquids. Applications include precursors to various plastics and pesticides.

Pirkle's alcohol is an off-white, crystalline solid that is stable at room temperature when protected from light and oxygen. This chiral molecule is typically used, in nonracemic form, as a chiral shift reagent in nuclear magnetic resonance spectroscopy, in order to simultaneously determine absolute configuration and enantiomeric purity of other chiral molecules. The molecule is named after William H. Pirkle, Professor of Chemistry at the University of Illinois whose group reported its synthesis and its application as a chiral shift reagent.

2,2-Di-2-furylpropane is a condensation product of furan and acetone. It is a relatively high boiling liquid and is a precursor to the rubber additive bis(tetrahydrofuryl)propane used in the manufacture of high vinyl content rubber for high performance tires.

3-Hydroxytetrahydrofuran is a colorless liquid with a normal boiling point of 179 °C and boiling at 88−89 °C at 17 mmHg, with density. 3-OH THF is a useful pharmaceutical intermediate. The chiral version of this compound is an intermediate to launched retroviral drugs.

Brookhart's acid is the salt of the diethyl ether oxonium ion and tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (BAr′4). It is a colorless solid, used as a strong acid. The compound was first reported by Volpe, Grant, and Brookhart in 1992.
In homogeneous catalysis, a C2-symmetric ligands usually describes bidentate ligands that are dyssymmetric but not asymmetric by virtue of their C2-symmetry. Such ligands have proven valuable in catalysis. With C2 symmetry, C2-symmetric ligands limit the number of possible reaction pathways and thereby increase enantioselectivity, at least relative to asymmetrical analogues. Most chiral ligands combine with metals to form chiral catalyst engages in a chemical reaction in which chirality is transfer to the reaction product.

















