
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. It is an isomer of another solvent, butanone.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is R−:C−R' or R=C: where the R represents substituents or hydrogen atoms.
In chemistry, an acetylide is a compound that can be viewed as the result of replacing one or both hydrogen atoms of acetylene (ethyne) HC≡CH by metallic or other cations. Calcium carbide is an important industrial compound, which has long been used to produce acetylene for welding and illumination. It is also a major precursor to vinyl chloride. Other acetylides are reagents in organic synthesis.

Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2).
An isocyanide is an organic compound with the functional group –N+≡C−. It is the isomer of the related nitrile (–C≡N), hence the prefix is isocyano. The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds.

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.

Polyphosphazenes include a wide range of hybrid inorganic-organic polymers with a number of different skeletal architectures with the backbone P-N-P-N-P-N-. In nearly all of these materials two organic side groups are attached to each phosphorus center. Linear polymers have the formula (N=PR1R2)n, where R1 and R2 are organic (see graphic). Other architectures are cyclolinear and cyclomatrix polymers in which small phosphazene rings are connected together by organic chain units. Other architectures are available, such as block copolymer, star, dendritic, or comb-type structures. More than 700 different polyphosphazenes are known, with different side groups (R) and different molecular architectures. Many of these polymers were first synthesized and studied in the research group of Harry R. Allcock.

In chemistry, a sulfenic acid is an organosulfur compound and oxoacid with the general formula R−S−OH. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids and sulfonic acids, respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide.

Metal–organic frameworks (MOFs) are a class of porous polymers consisting of metal clusters coordinated to organic ligands to form one-, two- or three-dimensional structures. The organic ligands included are sometimes referred to as "struts" or "linkers", one example being 1,4-benzenedicarboxylic acid (BDC).

Organozirconium chemistry is the science of exploring the properties, structure, and reactivity of organozirconium compounds, which are organometallic compounds containing chemical bonds between carbon and zirconium. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.
Covalent organic frameworks (COFs) are a class of porous polymers that form two- or three-dimensional structures through reactions between organic precursors resulting in strong, covalent bonds to afford porous, stable, and crystalline materials. COFs emerged as a field from the overarching domain of organic materials as researchers optimized both synthetic control and precursor selection. These improvements to coordination chemistry enabled non-porous and amorphous organic materials such as organic polymers to advance into the construction of porous, crystalline materials with rigid structures that granted exceptional material stability in a wide range of solvents and conditions. Through the development of reticular chemistry, precise synthetic control was achieved and resulted in ordered, nano-porous structures with highly preferential structural orientation and properties which could be synergistically enhanced and amplified. With judicious selection of COF secondary building units (SBUs), or precursors, the final structure could be predetermined, and modified with exceptional control enabling fine-tuning of emergent properties. This level of control facilitates the COF material to be designed, synthesized, and utilized in various applications, many times with metrics on scale or surpassing that of the current state-of-the-art approaches.
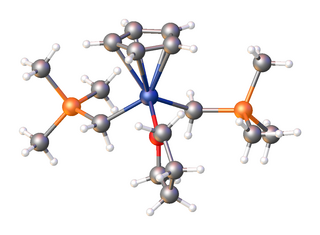
Organoscandium chemistry is an area with organometallic compounds focused on compounds with at least one carbon to scandium chemical bond. The interest in organoscandium compounds is mostly academic but motivated by potential practical applications in catalysis, especially in polymerization. A common precursor is scandium chloride, especially its THF complex.

Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.

In organic chemistry, the thiol-yne reaction is an organic reaction between a thiol and an alkyne. The reaction product is an alkenyl sulfide.
4,4′-Methylenedianiline (MDA) is an organic compound with the formula CH2(C6H4NH2)2. It is a colorless solid, although commercial samples can appear yellow or brown. It is produced on an industrial scale, mainly as a precursor to polyurethanes.

Copper hydride is an inorganic compound with the chemical formula CuHn where n ~ 0.95. It is a red solid, rarely isolated as a pure composition, that decomposes to the elements. Copper hydride is mainly produced as a reducing agent in organic synthesis and as a precursor to various catalysts.
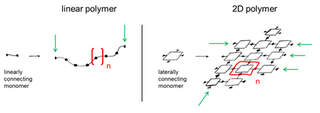
A two-dimensional polymer (2DP) is a sheet-like monomolecular macromolecule consisting of laterally connected repeat units with end groups along all edges. This recent definition of 2DP is based on Hermann Staudinger's polymer concept from the 1920s. According to this, covalent long chain molecules ("Makromoleküle") do exist and are composed of a sequence of linearly connected repeat units and end groups at both termini.

Transition metal carboxylate complexes are coordination complexes with carboxylate (RCO2−) ligands. Reflecting the diversity of carboxylic acids, the inventory of metal carboxylates is large. Many are useful commercially, and many have attracted intense scholarly scrutiny. Carboxylates exhibit a variety of coordination modes, most common are κ1- (O-monodentate), κ2 (O,O-bidentate), and bridging.

















